
Role of lncRNA FTX in invasion, metastasis, and epithelial-mesenchymal transition of endometrial stromal cells caused by endometriosis by regulating the PI3K/Akt signaling pathway
Introduction
The main symptoms of endometriosis (EMs) are pelvic pain and infertility caused by the implantation and growth of endometrial tissues with growth function outside the uterine cavity (1), which have serious adverse effects on the health and fertility of women of reproductive age (2). It has been reported that the incidence of EMs has significantly increased in recent years, affecting 10–15% of women of reproductive age; the incidence is as high as 25–40% among infertile women (3). It has been noted that ectopic endometrial tissues and cells have biologic functions of abnormal proliferation, invasion, metastasis, and apoptosis resistance, which are similar to malignant tumor cells (4,5). EMs is often treated by surgery in clinical practice, but is a difficult disease to cure, as it involves the thorough removal of tiny ectopic endometria. Because the pathogenesis of EMs remains unclear and its etiology cannot be completely eliminated, it is prone to recurrent attacks after surgery. Therefore, a better understanding of the pathogenesis of EMs is a priority.
Many studies have found that long non-coding RNAs (lncRNAs) cause a variety of biologic behaviors in EMs, which may be a key factor in the diagnosis or treatment of EMs (6,7). lncRNAs are a type of RNA molecule; they are longer than 200 nt and do not encode proteins (8) lncRNAs not only participate in the physiological process of body growth and development, but also play a crucial role in the incidence and development of diseases (9). Most lncRNAs cannot only regulate DNA replication, RNA transcription, and protein translation by complementary pairing with microRNAs or circRNAs, but also directly interact with target proteins to regulate protein activity (10). It has been noted that inhibiting the expression of lncRNA BRAF-activated non-coding RNA may inhibit the development of ectopic endometrial tissues by inhibiting the extracellular signal-regulated kinase/mitogen-activated protein kinase signaling pathway (11). Additionally, increasing the expression of lncRNA urothelial carcinoma associated 1 has the potential to reduce the risk of EMs (12). Therefore, lncRNA is important for regulating EMs development. Some studies have considered long non-coding RNA 5 prime to Xist (lncRNA FTX) a key lncRNA for normal uterine development (13), but it has not been reported whether lncRNA FTX is involved in regulating the development of EMs, and most of the previous studies on the relationship between lncrnas and endometriosis were conducted independently of clinical samples, or only explored the relationship between lncrnas expression and disease development. Therefore, based on the expression level of clinical samples, in the present study, we aimed to explore the effect and its mechanism of lncRNA FTX on the invasion, metastasis, and epithelial-mesenchymal transition (EMT) of endometrial stromal cells (ESC) caused by EMs in vitro so as to provide a theoretical basis for the diagnosis and treatment of EMs to find new therapeutic targets. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-20-6810).
Methods
Collection of tissue samples and clinical data
Patients with EMs who were diagnosed and surgically treated at Beijing Tongren Hospital from June 2018 to December 2019 (n=38) and healthy volunteers without EMs who underwent physical examination at our hospital during this period (n=20) were included the present study. The age of the included patients was recorded. Patients with the following conditions were included as samples: patients diagnosed with EMs by B-ultrasound and histopathology; patients who had not received hormone therapy within 3 months; patients with normal function of vital organs, such as heart, lung, liver, and kidney; and patients with normal menstruation. Patients with the following conditions were excluded: patients with complicated endocrine, autoimmune, and coagulation disorders and mental disorders; patients with gynecological diseases, such as complicated hysteromyoma and polycystic ovary syndrome; and lactating women, pregnant women, and women who were possibly pregnancy. All study procedures were approved by the Research Ethics Committee of Beijing Tongren Hospital. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients.
Cell culture and transfection
After removal of normal or ectopic endometrial tissue under aseptic conditions, the tissues were cut into 1-mm3 pieces, transferred to phosphate-buffered saline (PBS) containing 300 mg/mL of collagenase III and 40 mg/mL of deoxyribonuclease, and digested at 37 °C with oscillation at 2.5 g. After 45 min, the digestive juice was filtered through a 40-µm filter to remove the undigested tissues. The digested individual cells were separated and collected into centrifuge tubes, and the digestion was terminated by adding fetal bovine serum (FBS; Gibco, USA). After centrifuging at 800 rpm for 5 min at 4 °C, the supernatant was discarded, and F12 medium containing 10% serum was added to the resuspended cells. The cells were seeded in a 25-cm2 culture flask and placed in a 37 °C, 5% CO2 incubator (Thermo Fisher Scientific, Waltham, USA) to culture. ESC and ectopic endometrial stromal cells (EESC) were obtained. EESC were transfected with negative siRNA (si-NC), lncRNA FTX siRNA (si-FTX), pcDNA3.1 empty vector (pc-NC), and overexpressed pcDNA3.1-lncRNA FTX (pc-FTX) following the Lipofectamine 2000 transfection procedure of (Thermo, USA). EESC without any treatment were used as the control group. LncRNA FTX siRNA was designed and synthesized by Ruibo Bio (Guangzhou, China). The overexpressed pcDNA3.1-lncRNA FTX plasmid was synthesized by Addgene (USA).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) assay
Total RNA was extracted from tissues and cells using the TRIzol method (Thermo Fisher Scientific, Waltham, USA). The concentration and purity of RNA were detected with NanoDrop (Thermo Fisher Scientific, Waltham, USA). Reverse transcription into cDNA was performed following the random primer reverse transcription kit (Thermo, USA). The expression level of lncRNA FTX was detected following the instructions of the SYBR Green kit (TaKaRa, Japan). Six replicates were set up for the experiment using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. The experimental data obtained by qRT-PCR were used to calculate the relative expression of the target gene using the 2−ΔΔCt method. Primer sequences are shown in Table 1.

Full table
Cell proliferation rate detection by Cell Counting Kit-8 (CCK8)
A total of 5×104 EESC were seeded onto opaque 96-well plates, supplemented with 200 µL fresh F12 complete medium for 24 h. After the transfection of cells was completed, the cells were incubated for a further 24 h. After incubation, the fresh medium was changed, and the cells were cultured for 24, 48, and 72 h, respectively. A total of 10 µL CCK8 was added to each well, and the cells were incubated in the incubator for 1.5 h. The absorbance value (optical density) was measured with a microplate reader (450 nm).
Cell invasion detection by transwell assay
Twenty-four hours after transfection with 2×104 cells added to the upper chamber of the transwell chamber, 700 µL medium containing 20% FBS was added to the lower chamber of the transwell chamber. The transwell chamber was removed after 12–24 h of culture at 37 °C, 5% CO2, and was washed 3 times with PBS. The chamber was fixed with 1% glutaraldehyde for 30 min, washed with PBS, and dried; 0.1% crystal violet was then added and left for 12 h. The transwell chamber was then washed with PBS again. After the transwell chamber was dried, an upright microscope was used to observe 6–10 random fields. The number of positive cells in each field was recorded, and 3 fields were randomly chosen for photography and statistical analysis.
Cell migration detection by scratch assay
First, a transverse line was drawn with a ruler behind the 6-well plates with a marker, and then the cells transfected for 24 h were seeded onto the 6-well plates. After the cells were transfected separately, a 10 µL sterile lance tip was placed against a ruler and used to scratch perpendicular to the transverse line. Suspended cells and cell debris were removed by washing with PBS. Fresh serum-free medium was added, and after 24 h of culture, photographs were taken with an inverted microscope to record the experimental results. Image J software (National Institutes of Health, Bethesda, Maryland, USA) was used to calculate the scratch area.
Apoptosis detection by flow cytometry
Cells that had been transfected for 24 h were digested into centrifuge tubes using trypsin. After centrifugation at 71 g for 5 min at 4 °C, the cells were rinsed twice with pre-chilled sterile PBS. After centrifugation at 71g for 5 min at 4 °C, the cell concentration was adjusted to 5×105 cells/mL with PBS. A total of 200 µL cell suspension was taken and added with 10 µL Annexin V-fluorescein isothiocyanate and 10 µL propidium iodide (PI) solution at a concentration of 20 mg/L. This mixture was incubated for 10 min at room temperature under dark conditions. After adding 500 µL PBS, apoptosis was detected by flow cytometry.
Cell cycle detection by flow cytometry
Cells that had been transfected for 24 h were digested into centrifuge tubes using trypsin. After centrifugation at 71 g for 5 min at 4 °C, the cells were rinsed twice with pre-chilled sterile PBS. After centrifugation at 71 g for 5 min at 4 °C, the cells were resuspended by adding 1 mL pre-chilled ethanol with a volume fraction of 70%, and fixed overnight at 4 °C. After centrifugation, the cells were rinsed twice with pre-chilled sterile PBS. The cells were centrifuged for 5 min each time, and resuspended in 500 µL PBS. After adding PI solution with a final concentration of 50 µg/mL, the cells were incubated at room temperature for 15 min under dark conditions. The cell cycle was detected by flow cytometry.
Protein expression detection by Western blot
Cells that had been transfected for 24 h were rinsed twice with pre-chilled sterile PBS to remove PBS, and 80 µL cell lysate was added for 15 min at 4 °C. The lysed cells were transferred to a 200 µL centrifuge tube at a centrifugation of 15,984 ×g for 15 min at 4 °C; the cellular protein was then collected. The protein concentration was determined with a bicinchoninic acid (BCA) kit. In total, 20 µg protein was denatured by boiling in 1× loading buffer, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were then transferred to polyvinylidene difluoride membranes; 5% skimmed milk powder was then added to block for 1 h. Primary antibodies E-cadherin (Abcam, UK), vimentin (Abcam, UK), N-cadherin (Abcam, UK), zinc finger E-box binding homeobox 1 (ZEB1; Abcam, UK), p-PI3K (Abcam, UK), PI3K (Abcam, UK), p-Akt (Abcam, UK) and Akt (Abcam, UK) were added and later incubated overnight at 4 °C. The membranes were washed 3 times, and secondary antibodies were added to incubate for 1 h in the greenhouse. After washing the membrane a further 3 times, chemiluminescence reagents were added to develop the protein. Images were collected in a gel imaging system. Gray levels of the protein bands were analyzed using Image J software, with GAPDH as an internal reference to calculate the relative protein expression.
Statistical analysis
SPSS version 24.0 (SPSS, Chicago, IL, USA) was used for one-way analysis of variance and independent sample t-test analysis. The results were expressed as mean ± standard deviation. P<0.05 indicated statistical significance.
Results
lncRNA FTX underexpression in endometrial tissues and cells
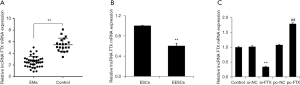
To determine the expression of lncRNA FTX in EMs, we collected endometrial tissues from patients with EMs and healthy volunteers. The expression of lncRNA FTX was found to be significantly reduced in the endometrial tissues of patients with EMs by qRT-PCR detection of clinical samples compared with the normal group (Figure 1A). Furthermore, we separated and cultured ESC and EESC. Compared with the ESC group, the expression level of lncRNA FTX was significantly reduced in the EESC group (Figure 1B). To further investigate the functional impact of the expression of lncRNA FTX on EESC, we overexpressed and interfered with the expression of lncRNA FTX in EESC. The results of the qRT-PCR detection indicated that the expression level of lncRNA FTX was significantly decreased in the cells of the si-FTX group compared with the si-NC group (Figure 1C). The expression level of lncRNA FTX was significantly increased in the cells of the pc-FTX group compared with the pc-NC group.
lncRNA FTX promotes proliferation, invasion, and migration of EESC
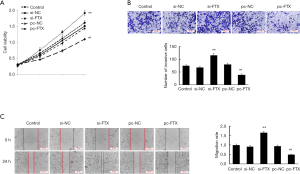
CCK8, transwell assay, and scratch assay were used to investigate the effect of lncRNA FTX on proliferation, invasion, and migration of EESC, respectively. According to the results of CCK8 assay (Figure 2A), the proliferation level of EESC was significantly increased after interfering with the expression of lncRNA FTX (P<0.05), and the proliferation level of EESC was significantly reduced after the overexpression of lncRNA FTX (P<0.05). The results of the transwell assay showed that the invasion of cells in the si-FTX group was significantly increased compared with the si-NC group (P<0.05), and the invasion of cells in the pc-FTX group was significantly decreased compared with the pc-NC group (P<0.05) (Figure 2B). The results of the scratch assay showed that the migration of cells in the si-FTX group was significantly increased compared with the si-NC group (P<0.05), and the migration of cells in the pc-FTX group was significantly decreased compared with the pc-NC group (P<0.05) (Figure 2C).
LncRNA FTX inhibits apoptosis in EESC and causes cell cycle arrest in the S phase
The flow cytometry results showed that the apoptotic rate of EESC significantly decreased after interfering with the expression of lncRNA FTX (Figure 3A). The proportion of cells in the G0/G1 phase significantly decreased, whereas the proportion of cells in the S phase increased (Figure 3B). The apoptotic rate of cells significantly increased after the overexpression of lncRNA FTX (Figure 3A). The proportion of cells in the G0/G1 phase significantly increased, whereas the proportion of cells in the S phase significantly decreased (Figure 3B).

Effect of lncRNA FTX on the expression of EMT and PI3K/Akt signaling pathway-related proteins in EESC
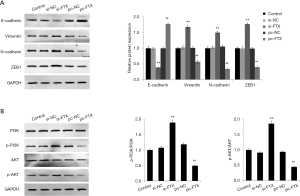
To further investigate the effect of the molecular mechanism of lncRNA FTX on the cellular function of EESC, we first detected the expression of EMT-related proteins in EESC of each group by Western blot. The results showed that the protein expression of E-cadherin significantly decreased, and the protein expression levels of vimentin, N-cadherin, and ZEB1 significantly increased in the cells of the si-FTX group compared with the si-NC group (P<0.05) (Figure 4A). Compared with the pc-NC group, the protein expression of E-cadherin significantly increased, and the protein expression levels of vimentin, N-cadherin, and ZEB1 significantly decreased in the cells of the pc-FTX group (P<0.05). It has been noted that PI3K/Akt signaling pathway activation is important for the development of EMs (14). Therefore, we detected the PI3K/Akt signaling pathway-related protein expression. The results showed that the expression of p-PI3K/PI3K and p-AKT/AKT significantly increased in the cells of the si-FTX group compared with the si-NC group (P<0.05) (Figure 4B). The expressions of p-PI3K/PI3K and p-AKT/AKT significantly decreased in the cells of the pc-FTX group compared with the pc-NC group (P<0.05). These results confirmed that lncRNA FTX is involved in regulating EMT transformation in EESC and in PI3K/Akt signaling pathway activation.
Discussion
Although there are many theories about the etiology of EMs, there is still no consensus on the pathogenesis of EMs. With continuous improvement in high-throughput sequencing and gene chip technology in recent years, studies are increasingly linking lncRNAs to the regulation of a cellular physiological metabolism. It has been noted that lncRNA FTX could inhibit the proliferation and metastasis of hepatocellular carcinoma by binding Minichromosome maintenance protein 2 and miR-374a (15), regulating cardiomyocyte apoptosis by targeting miR-29b-1-5p and Bcl2l2 (16), and inhibiting the proliferation and migration of osteosarcoma by regulating miR-320a/Thioredoxin Reductase 1 (17). lncRNA FTX is a key lncRNA in regulating the proliferation, migration, and apoptosis of cancer cells; however, the question remains as to whether lncRNA FTX has a corresponding effect on EMs, which is known as “undead cancer”. In the present study, using clinical sample tissues and in vitro cells, we found that lncRNA FTX was underexpressed in endometrial tissues and in EESC of patients in the EMs group. The results confirmed that lncRNA FTX was involved in the development of EMs. However, the possible function and mechanism of lncRNA FTX in EMs remain unclear.
The main difference between EESC and normal ESC is that the migration and invasion, as well as the growth and EMT of EESC, are enhanced (18). Therefore, we interfered with the expression of lncRNA FTX to study its effect on the molecular functions of EESC. After interference and overexpression of lncRNA FTX, we detected changes in the proliferation, invasion, and migration of EESC. After interfering with the expression of lncRNA FTX, the proliferation, invasion, and migration of EESC significantly increased, and apoptosis decreased. However, the proliferation, invasion, and migration of EESC significantly decreased after the overexpression of lncRNA FTX. The flow cytometry results showed that if the expression of lncRNA FTX increased, the apoptotic rate also increased, and lncRNA FTX would lead to the cell cycle arrest of EESC in the G0/G1 phase. However, recent studies have shown that the knockdown of lncRNA FTX inhibits the proliferation, migration, and invasion of renal cancer cells, and results in the cell cycle arrest of renal cancer cells in the G0/G1 phase (19). It has also been reported that lncRNA FTX promotes the progression of gastric cancer by targeting miR-215 (20). The findings of this experiment, combined with those of previous studies, indicate that the role of lncRNA FTX in the process of disease development is 2 sided.
It has been noted that PI3K/Akt signaling pathway activation is important for the development of EMs; it was not only involved in regulating the proliferation, invasion, and migration of EESC, but also affected the EMT of cells (14). However, inhibiting PI3K/Akt signaling pathway activity could have an inhibitory effect on EMs (21,22). EMT has been reported to be a key indicator of the invasive and metastatic process of ectopic endometria and the development of EMs (23). To investigate the effect of lncRNA FTX on EMT, as well as the mechanisms for EESC regulation, EMT-related proteins and PI3K/Akt signaling pathway-related proteins were detected by Western blot. We found that the overexpression of lncRNA FTX significantly increased the protein expression of E-cadherin, and decreased the protein expression of vimentin, N-cadherin, and ZEB1. The knockdown of lncRNA FTX may result in increased ratios of p-PI3K/PI3K and p-Akt/Akt, whereas the overexpression of lncRNA FTX decreased the ratios of p-PI3K/PI3K and p-Akt/Akt. These results suggest that the overexpression of lncRNA FTX can inhibit the activity of PI3K/Akt signaling pathway as well as the proliferation, invasion and EMT activity of EESCs in the clinical treatment, and prevent further deterioration of endometriosis.
Conclusions
The decreased expression of lncRNA FTX is associated with the development of EMs. The overexpression of lncRNA FTX can reduce EMs-induced proliferation, invasion, metastasis, and EMPT of ESC by inhibiting PI3K/Akt signaling pathway activity. Therefore, increasing the expression of lncRNA FTX could be used as a new treatment modality for EMs. However, because there are many cellular activities that lncRNA FTX can participate in, and because its interaction with target genes and target proteins is very complicated, the exact mechanism of lncRNA FTX in regulating the expression of EMT-related proteins and inhibiting PI3K/Akt signaling pathway activity needs to be further explored.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-6810
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-6810
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6810). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). All study procedures were approved by the Research Ethics Committee of Beijing Tongren Hospital. Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parasar P, Ozcan P, Terry KL. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr Obstet Gynecol Rep 2017;6:34-41. [Crossref] [PubMed]
- de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet 2010;376:730-8. [Crossref] [PubMed]
- Johnson NP, Hummelshoj L. World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum Reprod 2013;28:1552-68. [Crossref] [PubMed]
- Ferrero S, Vellone VG, Barra F. Pathophysiology of pain in patients with peritoneal endometriosis. Ann Transl Med 2019;7:S8. [Crossref] [PubMed]
- Nishida M, Nasu K, Ueda T, et al. Endometriotic cells are resistant to interferon-gamma-induced cell growth inhibition and apoptosis: a possible mechanism involved in the pathogenesis of endometriosis. Mol Hum Reprod 2005;11:29-34. [Crossref] [PubMed]
- Ahn SH, Singh V, Tayade C. Biomarkers in endometriosis: challenges and opportunities. Fertil Steril 2017;107:523-32. [Crossref] [PubMed]
- Luo H, Yang H, Lin Y, et al. LncRNA and mRNA profiling during activation of tilapia macrophages by HSP70 and Streptococcus agalactiae antigen. Oncotarget 2017;8:98455-70. [Crossref] [PubMed]
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem 2012;81:145-66. [Crossref] [PubMed]
- Backofen R, Vogel T. Biological and bioinformatical approaches to study crosstalk of long-non-coding RNAs and chromatin-modifying proteins. Cell Tissue Res 2014;356:507-26. [Crossref] [PubMed]
- Wu T, Du Y. LncRNAs: From Basic Research to Medical Application. Int J Biol Sci 2017;13:295-307. [Crossref] [PubMed]
- Shi S, Yang J, Fan W, et al. Effects of LncRNA MALAT1 on microangiopathy and diabetic kidney disease in diabetic rats by regulating ERK/MAPK signaling pathway. Minerva Med 2020;111:184-6. [Crossref] [PubMed]
- Huang H, Zhu Z, Song Y. Downregulation of lncrna uca1 as a diagnostic and prognostic biomarker for ovarian endometriosis. Rev Assoc Med Bras (1992) 2019;13:336-41. [PubMed]
- La Y, He X, Zhang L, et al. Comprehensive Analysis of Differentially Expressed Profiles of mRNA, lncRNA, and circRNA in the Uterus of Seasonal Reproduction Sheep. Genes (Basel) 2020;11:301. [Crossref] [PubMed]
- Madanes D, Bilotas MA, Bastón JI, et al. PI3K/AKT pathway is altered in the endometriosis patient's endometrium and presents differences according to severity stage. Gynecol Endocrinol 2020;36:436-40. [Crossref] [PubMed]
- Liu F, Yuan JH, Huang JF, et al. Long noncoding RNA FTX inhibits hepatocellular carcinoma proliferation and metastasis by binding MCM2 and miR-374a. Oncogene 2016;35:5422-34. [Crossref] [PubMed]
- Long B, Li N, Xu XX, et al. Long noncoding RNA FTX regulates cardiomyocyte apoptosis by targeting miR-29b-1-5p and Bcl2l2. Biochem Biophys Res Commun 2018;495:312-8. [Crossref] [PubMed]
- Huang S, Zhu X, Ke Y, et al. LncRNA FTX inhibition restrains osteosarcoma proliferation and migration via modulating miR-320a/TXNRD1. Cancer Biol Ther 2020;21:379-87. [Crossref] [PubMed]
- Liu Y, Lu C, Fan L, et al. MiR-199a-5p targets ZEB1 to inhibit the epithelial-mesenchymal transition of ovarian ectopic endometrial stromal cells via PI3K/Akt/mTOR signal pathway in vitro and in vivo. Reprod Sci 2020;27:110-8. [Crossref] [PubMed]
- He X, Sun F, Guo F, et al. Knockdown of long noncoding RNA FTX inhibits proliferation, migration, and invasion in renal cell carcinoma cells. Oncol Res 2017;25:157-66. [Crossref] [PubMed]
- Yang Y, Zhang J, Chen X, et al. LncRNA FTX sponges miR-215 and inhibits phosphorylation of vimentin for promoting colorectal cancer progression. Gene Ther 2018;25:321-30. [Crossref] [PubMed]
- Barra F, Ferro Desideri L, Ferrero S. Inhibition of PI3K/AKT/mTOR pathway for the treatment of endometriosis. Br J Pharmacol 2018;175:3626-7. [Crossref] [PubMed]
- Jing W, Yan W, Du X, et al. Sulforaphane Attenuates Endometriosis in Rat Models Through Inhibiting PI3K/Akt Signaling Pathway. Journal of Clinical and Experimental Medicine 2019;17:155-8.
- Zheng J, Luo X, Bao J, et al. Decreased Expression of HOXA10 May Activate the Autophagic Process in Ovarian Endometriosis. Reprod Sci 2018;25:1446-54. [Crossref] [PubMed]
(English Language Editor: R. Scott)


