Reconstruction of the hepatic artery using the superior mesenteric artery for liver transplantation
Introduction
Simultaneous pancreas and kidney transplantation is the most effective treatment option for end-stage diabetic nephropathy, which can eradicate diabetes mellitus as well as renal failure, and improve the long-term survival rate of diabetic patients compared to renal transplantation alone. However, the integrity of the common hepatic artery-pancreaticoduodenal artery (CHA-PDA) needs to be preserved during pancreatic repair in simultaneous pancreas and kidney transplantation to ensure the blood flow of the duodenum, and to reduce intestinal complications after simultaneous pancreas and kidney transplantation/pancreas transplantation. Therefore, the only arteries left to the donor liver are the proper hepatic artery (PHA) or the left/right hepatic artery (LHA/RHA), which often increases the difficulty of liver transplantation and requires revascularization in some cases. In previous experience, the donor's iliac artery/iliac vein were used for reconstruction (1). Our center used the superior mesenteric artery (SMA) and its branches to reconstruct the PHA and the LHA/RHA, and achieved good results.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-7200).
Methods
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Second Affiliated Hospital of Guangzhou Medical University (No. ChiCTR1900026543) and written informed consent was obtained from all patients.
Patient characteristics
Of the 17 donors, 9 had severe traumatic brain injury and 8 had cerebral hemorrhage. Donors were aged 20–51 years, 15 were male and 2 were female, with BMI ranging between 18.75–27.72. Total bilirubin ranged between 11.4–119.2 umol/L (median 33.7 µmol/L), alanine aminotransferase (ALT) ranged between 15–134 µ/L (median 32 µ/L), aspartate aminotransferase (AST) ranged between 19–98 µ/L (median 45 µ/L), and gamma-glutamyl transferase (GGT) ranged between 20–237 µ/L (median 57 µ/L).
The primary diseases of recipients were chronic acute liver failure in 5 cases, primary liver cancer in 5 cases, and chronic liver failure in 7 cases. The model for end-stage liver disease (MELD) scores (7–40 points) and Child-Pugh grades (A–C) were also evaluated.
Surgery process
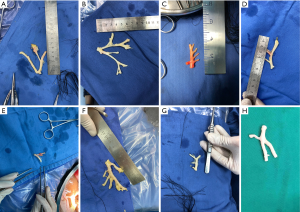
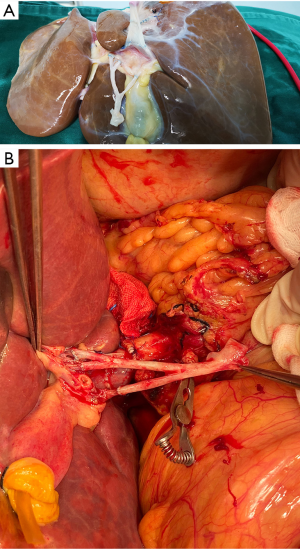
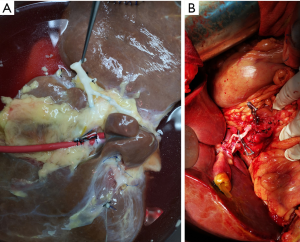
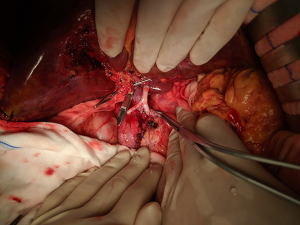
After routine en bloc acquisition of the donor liver, pancreas, and both kidneys, the abdominal aorta was cut between the renal artery and the SMA, the inferior vena cava was transected below the liver and above the right renal vein, the donor pancreas and liver were preserved, and the renal junction kidney group was trimmed. The surrounding tissues, including lymphatic vessels, the hepatic plexus, and surrounding connective tissue, were carefully dissected at the hepatic pedicle at the first porta hepatis. The common bile duct, hepatic portal vein, and PHA were freed from the right to the left, respectively. The distal part of the common bile duct was transected and doubly ligated with no. 7 and 4 silk sutures, the hepatic portal vein was transected approximately 1.5–2 cm away from the outlet of the pancreas, and the PHA was freed in the direction of the pancreas. The common hepatic artery and gastroduodenal arterial arch could be observed, the arterial variation was explored, then the integrity of the arterial arch was obtained. The PHA was transected approximately 0.5 cm away from the distal end of the arterial arch, the PHA transection was closed with a 5-0 atraumatic vascular suture, and the opening of the donor liver end was examined (Figure 1). If the hepatic artery was variant, the variant hepatic artery branches were divided separately (Figures 2 and 3). The SMA and its branches were obtained for liver reserve (Figure 4).
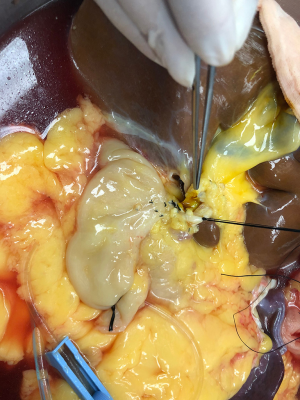
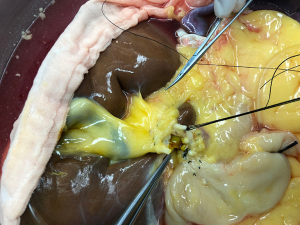
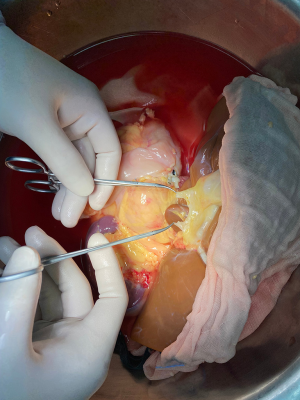
The donor liver was trimmed in vitro and revascularization was performed. After trimming the SMA and its branches and selecting the appropriate branches, the hepatic artery and SMA branches were end-to-end sutured with appropriate atraumatic vascular sutures, fixed at 4 points, then intermittently sutured. The reconstructed artery was then anastomosed end-to-end with the hepatic artery in vivo (Figures 5 and 6). If the PHA of the donor liver was not variable but short, end-to-end anastomosis was performed with the SMA and the PHA of the donor liver in vitro, and end-to-end anastomosis was performed between the SMA of the donor and the PHA of the recipient liver after the portal vein was opened (Figure 7).
Statistical analysis
SPSS20.0 software was used to process the data, and the measurement data of non-normal distribution was represented by the median.
Results
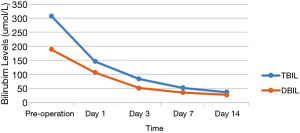
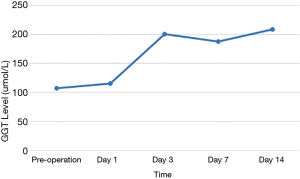
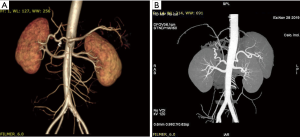
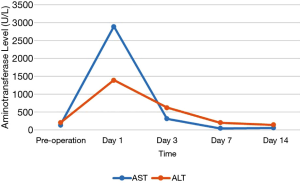
Of the 17 recipients, 14 were male and 3 were female, aged between 27 to 56 years. The MELD scores ranged from 1 to 35, and the Child-Pugh classifications were A to C. A total of 15 cases were modified piggyback liver transplantations, and 2 cases were classical piggyback liver transplantations. The intra-operative hemorrhagic volume was 300–6,000 mL, blood transfusion volume was 800–10,955 mL, warm ischemia time was 0 min, cold ischemia time was 5–8 hours, and the anhepatic period was 32–57 min, with an average of 44.5 min. The hospital stays ranged between 34–120 days, with an average of 61.3 days, and the ICU stays ranged between 1–8 days, with an average of 2.83 days. The patients were followed up for 1–96 months, and the prognosis was good. The color Doppler ultrasound of the transplanted liver showed that there were no obvious abnormalities of the blood flow signal of the hepatic artery. The computed tomography angiography (CTA) of the transplanted liver showed that the direction of the transplanted hepatic artery was normal, and the blood flow filling was normal (Figure 8). Postoperative bilirubin and transaminase levels decreased steadily (Figures 9-11). One patient developed hepatic artery stenosis (HAS) 3 months after surgery, and the stenotic segment disappeared after stent placement, whilst all other patients had no vascular complications. All patients had no biliary complications, and no acute/chronic rejection occurred.
Discussion
Good arterial perfusion is important for maintaining the function of the transplanted liver and reducing biliary complications. The incidence of hepatic artery complications has been reported in the literature to range from 2% to 9% in adult recipients and 7% to 26% in pediatric recipients (1,2). They mainly include hepatic artery thrombosis (HAT), HAS, as well as pseudoaneurysms (3,4). The hepatic arterial system is the most important source of the intrahepatic and extrahepatic biliary system and anastomosis in recipients. Therefore, abnormal hepatic artery hemodynamics after liver transplantation will directly lead to ischemic-type biliary lesions (ITBL), significantly increasing the occurrence of fatal biliary complications. Hernandez et al. (5) reported that out of 300 cases of liver transplantation, 12 cases had biliary stricture with patent hepatic artery without re-transplantation, while 9 cases had biliary tree necrosis or biliary stricture caused by HAT or HAS requiring re-transplantation. Torras et al. (6) reported that 16 out of 22 cases (72.7%) of ischemic biliary stricture required re-transplantation. Therefore, abnormal hepatic artery hemodynamics are independent risk factors for biliary complications.
It is generally accepted that the surgical suture technique is an important risk factor leading to HAT (7), which is also related to immune factors, donor liver quality, ischemic time, infection, and recipient coagulation status (8,9). Yang et al. (10) reported that complex graft hepatic artery reconstruction is also one of the risk factors for HAT.
The external iliac artery of the donor has been predominantly used in previous hepatic artery or branch reconstructions, however, it increases the incidence of HAT after pulse surgery (11). Del Gaudio et al. (12) reported that the incidence of postoperative HAT was 21.8% and 8.6% in recipients who had donor iliac artery bypass to reconstruct the hepatic artery and those who did not, respectively, whilst Warner et al. (13) reported rates of 11.1% and 4.4%, respectively. We used the donor SMA to reconstruct the hepatic artery, which produced a high success rate. Furthermore, we chose to reconstruct in vitro, thus reducing the surgical difficulty of in vivo reconstruction. Our results showed that 5.9% (1/17) of recipients with hepatic artery reconstruction using the SMA developed HAS after surgery, all of which were mild and did not affect liver function. Additionally, no arterial embolism occurred, which is comparable to the incidence of HAT in non-bypass recipients reported in the previous literature (12,13). Therefore, our transplantation method was effective and safe.
The SMA has distinct anatomical advantages. It is an important branch of the abdominal aorta, arising from the anterior wall of the abdominal aorta between the 12th thoracic vertebra and the 2nd lumbar vertebral body, with an origin approximately 10 mm below the celiac artery and located behind the pancreas. The SMA trunk descends posterior to the pancreaticocervicopancreatic junction, accompanies the superior mesenteric vein, and passes between the lower edge of the pancreatic neck and the duodenum. It crosses down to the level of the duodenum into the root of the small bowel mesentery. Xu and Tang (14) measured the diameter of the mesenteric artery on CT images. At the level where the left renal vein drains to the inferior vena cava, the mean diameter is 6.5 mm. At the level where the left renal vein drains to the plane 10 mm above the inferior vena cava, the mean diameter is 6.7 mm. At the level where the left renal vein drains 10 mm below the inferior vena cava, the diameter is 6.3 mm. The diameter of the SMA branches gradually decreases, and as there are many branches, they can match each hepatic artery or branch with different diameters. It therefore has advantages over the iliac artery in terms of branch matching, and also has good reproducibility.
Conclusions
Reconstruction of the hepatic artery with the SMA in vitro is a novel method of vascular reconstruction, which ensures blood flow of the hepatic artery, reduces the anastomosis difficulty of the arteries of the donor liver, and reduces the occurrence of vascular complications.
Acknowledgments
Funding: This work was supported by the Major Clinical Technology Program of Guangzhou, China (grant number 2019ZD12) and Medical Science and Technology Research Foundation of Guangdong Province, China (grant number A2020384).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-7200
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-7200
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-7200). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Second Affiliated Hospital of Guangzhou Medical University (No. ChiCTR1900026543) and written informed consent was obtained from all patients. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Silva MA, Jambulingam PS, Gunson BK, et al. Hepatic artery thrombosis following orthotopic liver transplantation: a 10-year experience from a single centre in the United Kingdom. Liver Transpl 2006;12:146-51. [Crossref] [PubMed]
- Miraglia R, Maruzzelli L, Caruso S, et al. Minimally invasive endovascular and biliary treatments of children with acute hepatic artery thrombosis following liver transplantation. Pediatr Radiol 2014;44:94-102. [Crossref] [PubMed]
- Pawlak J, Grodzicki M, Leowska E, et al. Vascular complications after liver transplantation. Transplant Proc 2003;35:2313-5. [Crossref] [PubMed]
- Zheng SS, Liang TB, Yu ZY, et al. Diagnosis and treatment of hepatic artery thrombosis after liver transplantation. Zhonghua Yi Xue Za Zhi 2004;84:1536-40. [PubMed]
- Hernandez Q, Ramirez P, Munitiz V, et al. Incidence and management of biliary tract complications following 300 consecutive orthotopic liver transplants. Transplant Proc 1999;31:2407-8. [Crossref] [PubMed]
- Torras J, Lladó L, Figueras J, et al. Biliary tract complications after liver transplantation: type, management, and outcome. Transplant Proc 1999;31:2406. [Crossref] [PubMed]
- Piardi T, Lhuaire M, Bruno O, et al. Vascular complications following liver transplantation: A literature review of advances in 2015. World J Hepatol 2016;8:36-57. [Crossref] [PubMed]
- Mourad MM, Liossis C, Gunson BK, et al. Etiology and management of hepatic artery thrombosis after liver transplantation. Liver Transpl 2014;20:713-23. [Crossref] [PubMed]
- Rajakannu M, Awad S, Ciacio O, et al. Intention-to-treat analysis of percutaneous endovascular treatment of hepatic artery stenosis after orthotopic liver transplantation. Liver Transpl 2016;22:923-33. [Crossref] [PubMed]
- Yang Y, Zhao JC, Yan LN, et al. Risk factors associated with early and late HAT after adult liver transplantation. World J Gastroenterol 2014;20:10545-52. [Crossref] [PubMed]
- Herrero A, Souche R, Joly E, et al. Early Hepatic Artery Thrombosis After Liver Transplantation: What is the Impact of the Arterial Reconstruction Type? World J Surg 2017;41:2101-10. [Crossref] [PubMed]
- Del Gaudio M, Grazi GL, Ercolani G, et al. Outcome of hepatic artery reconstruction in liver transplantation with an iliac arterial interposition graft. Clin Transplant 2005;19:399-405. [Crossref] [PubMed]
- Warner P, Fusai G, Glantzounis GK, et al. Risk factors associated with early hepatic artery thrombosis after orthotopic liver transplantation-univariable and multivariable analysis. Transpl Int 2011;24:401-8. [Crossref] [PubMed]
- Chuanbin X, Fagong T, Radiology DO. Anatomical study of the superior mesenteric artery on CT. J Regional Anat Operative Surg 2003;12:8-9.