
Safety of different carotid artery revascularization strategies in the coronary artery bypass graft population: study protocol for a systematic review and network meta-analysis
Introduction
Atherosclerosis is a systemic vascular disorder involving multiple arterial beds, including carotid and coronary arteries. Carotid intima-media thickness has been reported to be a good surrogate marker for coronary artery disease (CAD) (1). In previous studies, it was estimated that as many as 30% of CAD patients suffered from concomitant carotid artery occlusive diseases (CAOD) (2), and 23% of coronary artery bypass graft (CABG) patients had significant CAOD (carotid artery diameter stenosis >50% or carotid artery occlusion) (3). Also, 31.5% of patients who received carotid endarterectomy (CEA) and 56.5% of patients who underwent carotid artery stenting (CAS) were reported to have newly diagnosed significant CAD (4,5).
Carotid artery stenosis was thought to be an independent predictor of stroke in patients undergoing CABG (3,6). According to Naylor’s research, carotid artery disease is potentially responsible for up to 40% of post-CABG strokes (7). Furthermore, 18% of all cardiovascular-related fatalities were reported to be attributable to stroke caused by carotid artery atherosclerosis (8,9). Recent decades, CEA and CAS have been the main surgical techniques used for the management of significant CAOD. In the treatment of significant CAOD, the effectiveness of these two techniques is known to be comparable. However, the optimal technique and timing of carotid artery revascularization in CABG patients with coexisting significant CAOD is still controversial (10). Some studies have shown that combination strategies in which CEA or CAS is performed simultaneously with CABG are best (11-14), while other studies have favored staged strategies involving CEA or CAS with CABG, with the procedures being performed on different days (15-17). Moreover, some studies have treated different staged strategies using the same technique (CEA or CAS) but with different timings (before or after CABG) as one strategy (12,13,18).
The conflicting evidence means that a clear hierarchy of the available strategies based on the risk of perioperative adverse events, such as stroke, myocardial infarction (MI), and death, cannot be generated. To date, no published network meta-analysis (NMA) has combined direct and indirect evidence for these surgical approaches. Given the comparable effectiveness of CEA and CAS in treating significant CAOD, and the heterogeneity of previous original studies, we plan to perform a Bayesian NMA using a random-effects model to comprehensively compare and rank different surgical options for the management of CABG patients with concomitant significant CAOD based on their perioperative safety.
Before proceeding with the actual review, we chose to develop a protocol for the following reasons: (I) no published protocol of a Bayesian NMA currently exists for this topic; (II) a protocol would facilitate careful planning and, thereby, potential problems could be anticipated; (III) the plan would be explicitly documented before the review is started, enabling others to compare the protocol and the completed review (i.e., to identify selective reporting), to replicate review methods if desired, and to evaluate the validity of the planned methods; (IV) arbitrary decision-making with respect to the inclusion criteria and extraction of data would be prevented; (V) a protocol could help to reduce duplication of efforts and enhance collaboration (19). We prepared the protocol in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4451).
Methods
We have registered on the international prospective register of systematic reviews, PROSPERO, to publish our study protocol. This protocol was prepared according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 (19). The meta-analysis will be conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (20) and the PRISMA extension statement incorporating network meta-analyses of healthcare interventions (21).
The data that will be used in this NMA are not individual or private. Therefore, this NMA does not require ethical approval or informed consent. The results of this NMA will be published in a peer-reviewed journal.
Inclusion criteria for studies
Studies meeting the ‘PICOS’ structure described below will be included. Studies meeting any of the exclusion criteria described below will be excluded.
Types of participants (P)
Eligible patients are candidates for both CABG and carotid artery revascularization (CEA or CAS).
Type of interventions (I)
Six surgical strategies using the two techniques (CEA and CAS) with three different timings (before, after, or combined with CABG) will be evaluated, including: combined-CEA-CABG, staged CEA before CABG (staged-CEA/CABG), staged CABG before CEA (staged-CABG/CEA), combined-CAS-CABG, staged CAS before CABG (staged-CAS/CABG), and staged CABG before CAS (staged-CABG/CAS).
Comparison (C)
Combined CEA-CABG or another surgical strategy described above will be considered the control. Studies comparing the primary outcomes (described below) of two or more of these surgical strategies will be included.
Type of outcomes (O)
The primary outcomes will be the probability of perioperative stroke and the probability of perioperative death of each strategy. The secondary outcome will be the probability of perioperative MI. ‘Perioperative’ refers to the period from the initiation of a surgical strategy to approximately 30 days after the completion of that strategy. If 30-day post-operative results are unavailable, we will give preference to the time point closest to 30 days after the completion of a strategy. Stroke will be defined as a new or worsening focal neurological event that persisted for more than 24 hours. MI will be defined using at least two of the following criteria: typical chest pain lasting >20 min; serum levels of creatine kinase, creatine kinase myocardial band (CK-MB), or troponin at least twice the upper limit of the normal range; new Q-waves on at least two adjacent derivations.
Type of studies (S)
Prospective randomized controlled trials (RCTs), quasi-randomized trials (e.g., patients allocated using alternate days of the week), prospective, and retrospective cohort studies will be included.
Exclusion criteria
Studies with sample sizes smaller than 10 will be excluded. We will also exclude non-English articles, reviews, comments, short surveys, letters, conference abstracts, editorials without full-text, and studies reporting unextractable data.
Data sources and search strategies
Literature searches will be conducted covering articles published in four databases (PubMed, the Cochrane Central Register of Controlled Trials, Web of Science, and Embase) between January 1, 1989 and December 31, 2019. The searches will use the following Medical Subject Headings (MeSH) and text words: “ischemic heart disease*” or “myocardial ischemia*” or “coronary artery disease*” or “coronary artery arterioscleroses” or “coronary artery arteriosclerosis” or “coronary artery atherosclerosis” or “coronary atheroscleroses” or “coronary arterioscleroses” or “coronary arteriosclerosis”, and “carotid stenosis” or “carotid stenoses” or “carotid artery stenoses” or “carotid artery stenosis” or “carotid artery narrowing*” or “carotid artery plaques” or “carotid ulcer*”, and “coronary artery bypass*” or “coronary artery bypass surgery” or “coronary artery bypass surgeries” or “coronary artery bypass graft*” or “aortocoronary bypass*”, and “CEA” or “carotid endarterectomy” or “carotid endarterectomies” or “carotid artery stent*” or “carotid artery stenting”. Search results will be limited to articles published in English. There are no restrictions on the type of publication. The reference lists of eligible studies and relevant review articles will be searched to avoid missing any other potentially suitable articles.
Study selection
Four investigators (YS and SJ; SX and JS) will work in pairs to independently assess the titles and abstracts of the retrieved studies. The studies that are potentially eligible for inclusion will be selected for full-text screening and subsequently assessed for adequacy according to the proposed PICOS. The four investigators will also scrutinize the references of the included studies and relevant reviews. Any disagreements will be resolved by a consensus meeting with another three investigators (CL, ZH, DG).
Data extraction
Two reviewers (YS and SJ) will independently extract the key trial parameters using a standardized data abstraction form. If necessary, relevant authors will be contacted to supplement ambiguous or incomplete data from the original papers. The standardized data extraction form will include the trial characteristics, patient characteristics, intervention details, and outcome measures (Table 1).
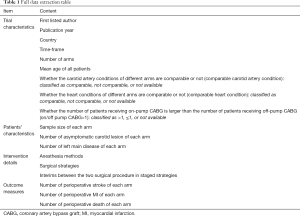
Full table
Assessment of risk of bias in the included studies
Four investigators (YS and SJ; SX and JS) will work in pairs to independently assess the risk of bias in the included studies. The risk of bias in included randomized control trials will be assessed using the Cochrane risk-of-bias tool. The risk of bias in included non-randomized studies will be assessed using the ROBINS-I tool (22). Any discrepancies will be resolved by a panel of other reviewers within the review team (CL, ZH, and DG).
Data synthesis and analysis
Data from the included studies will be synthesized. We will first pool direct evidence for each intervention comparison by performing pairwise meta-analysis. Direct and indirect evidence for each comparison will be integrated in the subsequent NMA.
Pairwise meta-analysis
We will calculate the average odds ratio (OR) and 95% confidence interval (CI) for each outcome with the random-effects model using R package meta (version 4.9-7) in R Project for Statistical Computing (version 3.6.1, RRID:SCR_001905) (23). Statistical heterogeneity in each pairwise comparison will be assessed with the I2 statistic and P value. A two-sided P value <0.05 will be considered statistically significant.
NMA
We will use the WinBUGS software package (V.1.4.3, MRC Biostatistics Unit, Cambridge, UK, RRID:SCR_018516) to perform NMA in a Bayesian framework in order to compare the relative outcomes of different surgical strategies from the median of the posterior distribution. For multi-arm trials, random-effects models will be adopted, using the binomial likelihood for dichotomous outcomes, uninformative prior distributions for the treatment effects, and a minimally informative prior distribution for the common heterogeneity variance (τ2) (24,25). For the categorical outcomes, median ORs with 95% credible intervals (CrIs) will be obtained with the 2.5th and 97.5th percentiles of the posterior distribution. If 95% CrIs do not include 0, a two-sided P<0.05 will be assumed at conventional levels of statistical significance. 95% Predictive intervals (PrIs) of these ORs will also be calculated to obtain a predictive distribution for the true effect in a new study as recommended. Pooled estimates will be obtained using the Markov Chains Monte Carlo (MCMC) method. For every pairwise comparison in a network, a Bayesian P value of (predictive) OR <1 will be calculated by counting the proportion of MCMC iterations in which (predictive) OR <1 (26). Three Markov chains will be run simultaneously with different arbitrarily-chosen initial values. Convergence of models will be ensured by visual inspection of the three chains and after considering the Brooks-Gelman-Rubin diagnostic (27). Convergence will be considered adequate after 20,000 samples have been run for each chain; these samples will be discarded as ‘burn-in’, and posterior summaries will be based on 80000 subsequent simulations for each chain. Both the consistency and inconsistency models will be fitted to each outcome. Deviance information criteria (DIC), and common heterogeneity variance (τ2) of the different models will be monitored and compared to check the fit of a model. Probability values will be summarized and reported as surface under the cumulative ranking curve (SUCRA) (28). Global heterogeneity will be assessed using the I2 statistic with the GeMTC R package (version 0.8-2) by assuming a common heterogeneity parameter for all comparisons in a model (29).
Subgroup analysis, sensitivity analysis, and meta-regression
If the necessary data are available, we will evaluate the robustness of the estimated treatment effects in subgroup analyses using a model with a single interaction term, β, according to the following extracted covariates in Table 1: comparable carotid artery condition (comparable, not comparable, or not available), comparable heart condition (comparable, not comparable, or not available), on/off pump CABG>1(>1, ≤1, or not available). Sensitivity network meta-analyses will also be conducted for each outcome by omitting trials with high risk of bias. Network meta-regression models with mean age, study timeframe, and year of publication will be carried out respectively to examine their impact on the network estimates and the underlying transitivity assumption of the NMA.
Trial-specific baseline risk is a proxy for important but undetected patient-level characteristics that produce significant clinical heterogeneity. Generally, unmeasured confounders cannot be accounted for completely, despite efforts to comprehensively adjust for all clinical variables. Therefore, a meta-regression model with trial-specific baseline risk using a Bayesian approach has been recommended as a more appropriate method for investigating the relationship between treatment effect and underlying baseline risk across trials in meta-analyses and adjusting for baseline confounders (30,31). Given the complex baseline characteristics of CABG patients with coexisting CAOD, we will apply the meta-regression model with trial-specific baseline risk to ulteriorly confirm the robustness of the estimated treatment effects.
Other analyses
We will perform further analyses with network package (version 1.5) in STATA (version 15.1, RRID:SCR_012763) (32). A global inconsistency test of the network will be conducted by fitting a full-design-by-treatment interaction model for each outcome (33). If any loops are connecting three or more interventions, the node-splitting method will be used to calculate inconsistency between direct and indirect evidence of each loop (34). The ratio of two odds ratios (RoR) and 95% CI from direct and indirect evidence in a closed loop will also be calculated to identify possible sources of inconsistency (35).
Assessment of publication bias
A comparison-adjusted funnel plot will be plotted to detect the presence of any dominant publication bias in the NMA (35).
Quality of evidence
We will assess the certainty of evidence contributing to each network estimate using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework to reflect specific issues from the NMA (36). Based on the five categories (study limitation, indirectness, inconsistency, imprecision, and publication bias), the quality of evidence of the NMA will be rated as high, moderate, low, or very low.
Discussion
Our Bayesian NMA will rank various surgical strategies for managing CABG patients with concomitant significant CAOD based on direct and indirect evidence of their perioperative safety. To the best of our knowledge, this will be the first Bayesian NMA performed to explore the perioperative safety of various surgical strategies in CABG patients with concomitant significant CAOD. This Bayesian NMA will provide a general overview and useful evidence of the perioperative safety of different surgical strategies for the management of CABG patients with coexisting significant CAOD. The results of this Bayesian NMA will also have important implications for clinical practice and further research.
Acknowledgments
We would like to thank Lu-Cheng Zhu (Department of Oncology, Hangzhou Cancer Hospital), Xiao Tang and Min Zhou (Department of Vascular Surgery, Institute of Vascular Surgery, Zhongshan Hospital, Fudan University), and Zhi-De Hu (Department of Laboratory Medicine, the Affiliated Hospital of Inner Mongolia Medical University) for their valuable advice.
Funding: This work was supported by grants from the Project of Shanghai Municipal Commission of Health and Family Planning (No. 19411966900), the Shanghai Sailing Program (Grant No. 19YF1406100), the National Natural Science Foundation of China (No. 81970408), and the Zhejiang Province Public Welfare Technology Application Research Project (No. LGF18H060012). The funders had no role in the study design or development of the protocol.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. available at: http://dx.doi.org/10.21037/atm-20-4451
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure forms(available at: http://dx.doi.org/10.21037/atm-20-4451). The authors have no conflicts of interest to declare.
Ethical Statements: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The data that will be used in this NMA are not individual or private. Therefore, this NMA does not require ethical approval or informed consent. The results of this NMA will be published in a peer-reviewed journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tran LTT, Park HJ, Kim HD. Is the carotid intima-media thickness really a good surrogate marker of atherosclerosis? J Atheroscler Thromb 2012;19:680-90. [Crossref] [PubMed]
- Steinvil A, Sadeh B, Arbel Y, et al. Prevalence and predictors of concomitant carotid and coronary artery atherosclerotic disease. J Am Coll Cardiol 2011;57:779-83. [Crossref] [PubMed]
- Santarpino G, Nicolini F, De Feo M, et al. Prognostic Impact of Asymptomatic Carotid Artery Stenosis in Patients Undergoing Coronary Artery Bypass Grafting. Eur J Vasc Endovasc Surg 2018;56:741-8. [Crossref] [PubMed]
- Illuminati G, Schneider F, Greco C, et al. Long-term results of a randomized controlled trial analyzing the role of systematic pre-operative coronary angiography before elective carotid endarterectomy in patients with asymptomatic coronary artery disease. Eur J Vasc Endovasc Surg 2015;49:366-74. [Crossref] [PubMed]
- Sulženko J, Paluszek P, Machnik R, et al. Prevalence and predictors of coronary artery disease in patients undergoing carotid artery stenting. Coron Artery Dis 2019;30:204-10. [Crossref] [PubMed]
- Cheng Y, Gao J, Wang J, et al. Risk Factors for Carotid Artery Stenosis in Chinese Patients Undergoing Coronary Artery Bypass Graft Interventions. Medicine (Baltimore) 2015;94:e1119. [Crossref] [PubMed]
- Naylor AR. How robust is the evidence supporting prophylactic carotid endarterectomy in patients undergoing coronary bypass? Acta Chir Belg 2004;104:626-9. [Crossref] [PubMed]
- Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119:480-6. [Crossref] [PubMed]
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation 2011;123:e18-209. [Crossref] [PubMed]
- Naylor AR. Managing patients with symptomatic coronary and carotid artery disease. Perspect Vasc Surg Endovasc Ther 2010;22:70-6. [Crossref] [PubMed]
- Versaci F, Reimers B, Del Giudice C, et al. Simultaneous Hybrid Revascularization by Carotid Stenting and Coronary Artery Bypass Grafting The SHARP Study. JACC Cardiovasc Interv 2009;2:393-401. [Crossref] [PubMed]
- Gopaldas RR, Chu D, Dao TK, et al. Staged versus synchronous carotid endarterectomy and coronary artery bypass grafting: analysis of 10-year nationwide outcomes. Ann Thorac Surg 2011;91:1323-9; discussion 1329. [Crossref] [PubMed]
- Sharma V, Deo SV, Park SJ, et al. Meta-analysis of staged versus combined carotid endarterectomy and coronary artery bypass grafting. Ann Thorac Surg 2014;97:102-9. [Crossref] [PubMed]
- Avdeyev A, Mendykulov S, Tabarov A, et al. Simultaneous coronary artery bypass grafting and carotid endarterectomy. Value in Health 2016;19:A44. [Crossref]
- Chan JSK, Shafi AMA, Grafton-Clarke C, et al. Systematic review and meta-analysis of staged vs simultaneous coronary artery bypass grafting surgery and carotid endarterectomy. J Cardiac Surg 2019;34:803-13. [Crossref]
- Giannopoulos S, Texakalidis P, Charisis N, et al. Synchronous Carotid Endarterectomy and Coronary Artery Bypass Graft versus Staged Carotid Artery Stenting and Coronary Artery Bypass Graft for Patients with Concomitant Severe Coronary and Carotid Stenosis: A Systematic Review and Meta-analysis. Ann Vasc Surg 2020;62:463-73.e4. [Crossref] [PubMed]
- Tzoumas A, Giannopoulos S, Texakalidis P, et al. Synchronous versus Staged Carotid Endarterectomy and Coronary Artery Bypass Graft for Patients with Concomitant Severe Coronary and Carotid Artery Stenosis: A Systematic Review and Meta-analysis. Ann Vasc Surg 2020;63:427-38.e1. [Crossref] [PubMed]
- Timaran CH, Rosero EB, Smith ST, et al. Trends and outcomes of concurrent carotid revascularization and coronary bypass. J Vasc Surg 2008;48:355-60; discussion 360-1. [Crossref] [PubMed]
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann Intern Med 2015;162:777-84. [Crossref] [PubMed]
- Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019;22:153-60. [Crossref] [PubMed]
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. [Crossref] [PubMed]
- Salanti G, Higgins JP, Ades AE, et al. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279-301. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009;172:137-59. [Crossref] [PubMed]
- Brooks SP, Gelman A. General Methods for Monitoring Convergence of Iterative Simulations. J Comput Graph Stat 1998;7:434-55.
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. [Crossref] [PubMed]
- Neupane B, Richer D, Bonner AJ, et al. Network meta-analysis using R: a review of currently available automated packages. PLoS One 2014;9:e115065. [Crossref] [PubMed]
- Achana FA, Cooper NJ, Dias S, et al. Extending methods for investigating the relationship between treatment effect and baseline risk from pairwise meta-analysis to network meta-analysis. Stat Med 2013;32:752-71. [Crossref] [PubMed]
- Dias S, Sutton AJ, Welton NJ, et al. NICE DSU Technical Support Document 3: Heterogeneity: Subgroups, Meta-Regression, Bias and Bias-Adjustment. National Institute for Health and Clinical Excellence, 2011.
- White IR. Network meta-analysis. Stata J 2015;15:951-85. [Crossref] [PubMed]
- Higgins JP, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98-110. [Crossref] [PubMed]
- Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. [Crossref] [PubMed]
- Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. [Crossref] [PubMed]
- Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS One 2014;9:e99682. [Crossref] [PubMed]


