
Effect of liver injury on prognosis and treatment of hospitalized patients with COVID-19 pneumonia
Introduction
In December 2019, pneumonia cases of unknown etiology were reported in the city of Wuhan, Hubei Province, China. The pathogen responsible for the outbreak was identified as a novel enveloped RNA beta coronavirus (1,2). This virus was named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and the disease it causes has become known as coronavirus disease 2019 (COVID-19) (3,4). SARS-CoV-2 shares approximately 80% of the genetic sequence with severe acute respiratory syndrome coronavirus (SARS-CoV) (5). SARS-CoV-2 is more contagious and has a higher rate of infectivity than SARS-CoV. As the COVID-19 outbreak spread rapidly across the world, the World Health Organization (WHO) declared it a public health emergency of international concern (6). As of September 22, 2020, more than 30,000,000 confirmed COVID-19 cases had been reported globally, and more than 960,000 people had died of the infection (7).
Although SARS-CoV mainly attacked the respiratory system, liver injury was reported in up to 60% of patients with SARS (8). SARS-CoV-2 shares the same viral entry receptor as SARS-CoV, angiotensin-converting enzyme 2 (ACE2), mainly in the respiratory tract mucosa or alveolus (4), and many cases of liver injury have been reported in patients with COVID-19 during disease progression (9,10). In a large cohort study conducted in China, which included 1,099 COVID-19 patients from 552 hospitals in 31 provinces or provincial municipalities, only 2.3% of cases had pre-existing liver illnesses; however, elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were observed in 21.3% and 22.2% of cases, respectively (1).
Therefore, liver injury does not appear to be uncommon in patients with COVID-19. When patients with COVID-19 have liver injury, especially when liver injury becomes more severe during treatment, clinicians need to carefully weigh the benefits and adverse effects of medications used, and even amend the therapeutic strategy and shorten the duration of drug therapy. However, few studies have focused on the effect liver injury has on the prognosis and treatment of COVID-19.
This retrospective study aimed to investigate the prevalence of liver injury in confirmed and hospitalized COVID-19 pneumonia cases, and evaluate its potential impact on the prognosis and treatment of this disease. The following article is presented in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4850).
Methods
Study design and participants
In this retrospective, single-center study, hospitalized patients with laboratory-confirmed COVID-19 pneumonia were recruited from wards 20 and 22 at the Eastern Branch of People’s Hospital of Wuhan University (Wuhan, Hubei, China) between January 31 and February 29, 2020. This hospital was one of the designated hospitals in Wuhan for the treatment of severe adult COVID-19 cases and was supported by a medical assistance team from Zhongshan Hospital of Fudan University, Shanghai, China. All patients with COVID-19 pneumonia enrolled in this study were diagnosed according to the guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the National Health Commission (Trial Version 7) (11). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of People’s Hospital of Wuhan University (NO. WDRY-2020-K048). Due to the study’s retrospective nature, the requirement for individual consent was waived.
Procedures
The following data were obtained from the patients’ medical records: epidemiological and demographic information, coexisting illnesses, symptoms, signs, laboratory findings, X-ray or chest computed tomographic (CT) scan images, treatment, and outcome. The final date of follow-up was March 31, 2020. Radiologic and laboratory tests including liver biochemical parameters and nasopharyngeal swab examination were performed within 24 hours after admission. A confirmed case of COVID-19 was defined as a positive result on real-time reverse transcription polymerase chain reaction (RT-PCR) assay of nasopharyngeal swab specimens (10).
According to the guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the National Health Commission (Trial Version 7) (11) and the American Thoracic Society guidelines for community-acquired pneumonia (12), at the time of admission, cases were defined as severe with a respiratory rate >30 breaths/min, blood oxygen saturation (SpO2) <93% on room air, or oxygenation index (PaO2/FiO2) ≤300 mmHg. Patients who did not meet one of these criteria were classified as non-severe cases.
Liver injury was indicated when the levels of ALT, AST, or γ-glutamyl transferase (GGT) exceeded the upper limit of the normal laboratory reference range.
The primary composite end-point event was the use of mechanical ventilation or death, which has been used to assess the severity of other serious infectious diseases, such as H7N9 infection (1,13).
Statistical analysis
Continuous variables were expressed as median and interquartile range (IQR), and categorical variables were described as counts and percentages. For continuous variables, the medians were compared using independent samples t tests when the data were normally distributed, and Mann-Whitney U tests were used if the data were not normally distributed. Proportions for categorical variables were compared using the chi-squared test or Fisher’s exact test where appropriate. The degree of association between the parametric variables was measured using Pearson’s correlation or Spearman’s correlation analysis, and a step-wise logistic regression analysis was conducted to explore the risk factors for the primary composite end-point event. All statistical analyses were performed with the SPSS 26.0 (SPSS Inc., Chicago, IL, USA) software package. A two-tailed P value of <0.05 was considered to be statistically significant.
Results
Clinical characteristics of enrolled patients
Between January 31 and February 29, 2020, 115 patients with COVID-19 pneumonia were admitted. Six of these patients were excluded from this study due to having incomplete medical history or laboratory data. The demographic, clinical and laboratory characteristics of the enrolled patients are shown in Table 1. The patients had a median age of 63 years (range, 30–87 years), and 46.8% were male. At admission, the degree of COVID-19 pneumonia was categorized as non-severe in 53 patients (48.6%) and severe in 56 patients (51.4%). Among the admitted patients, 46.8% had at least one coexisting illness (e.g., hypertension or diabetes mellitus). Additionally, one patient (0.9%) had chronic hepatitis B and had been taking entecavir for more than a year; and this patient had normal liver function test at admission. No other cases of chronic liver disease were reported among the enrolled patients.

Full table
Thirty-nine (35.8%) patients presented with liver injury, which manifested in 61.5% of these cases as an elevated level of ALT or AST accompanied simultaneously by an increase in the level of GGT. An elevated level of ALT or AST alone was observed in a further 10 patients (25.6%), and mono-elevated levels of GGT were found in 5 patients (12.8%). Only 3 patients (7.7%) had elevated total bilirubin, and the median level was at 10.60 mmol/L (range, 4.50–65.00 mmol/L).
The 109 patients enrolled had a median hospital stay of 30.50 days (range, 6–55 days). During hospitalization, 37 patients (33.9%) were treated with glucocorticoids. Primary composite end-point events occurred in 21 patients (19.3%), including 5 patients (4.6%) who underwent invasive mechanical ventilation, and 5 patients (4.6%) who died. One patient (0.9%) underwent extracorporeal membrane oxygenation, but died after 26 days.
Comparison between patients with non-severe and severe disease
Patients with severe disease were older than those with non-severe disease (P=0.049). Liver injury was more prevalent in patients with severe COVID-19 pneumonia than in those with non-severe disease (46.4% vs. 24.5%, P=0.017), with severe cases having higher levels of AST and GGT and a lower level of albumin. However, no significant differences were observed in the levels of ALT, total bilirubin, and prothrombin time or international normalized ratio (INR) between the two groups of patients (all P>0.05). Additionally, inflammatory markers including white blood cell count, the percentage of neutrophil granulocytes, and C-reactive protein levels were all significantly increased in patients with severe disease, whereas the percentage of lymphocytes was significantly decreased (all P<0.05) (Table 2).
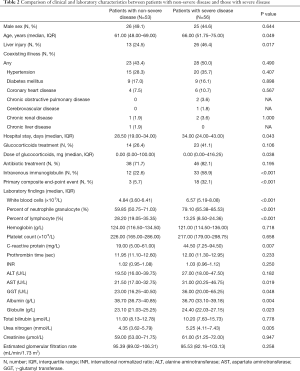
Full table
All of the admitted patients were administered the antiviral drug Abidor. During hospitalization, 23 patients with severe disease and 14 patients with non-severe disease were treated with glucocorticoids (41.1% vs. 26.4%, P=0.106); however, the patients with severe disease received far more doses of glucocorticoids than those with non-severe disease (P=0.038). The number of patients who received antibiotic treatment did not differ between the two groups (P=0.195), but more patients with severe disease received intravenous immunoglobulin (58.9% vs. 22.6%, P<0.001). Primary composite end-point events occurred more in patients with severe disease than in those with non-severe disease (32.1 vs. 5.7%, P<0.001).
Associated risk factors of liver injury at admission
The correlations between liver injury and other variables at admission were further analyzed. The occurrence of liver injury was positively correlated with the severity of COVID-19 infection and inflammatory markers including white blood cell count, the percentage of neutrophil granulocytes, and C-reactive protein levels, but negatively correlated with the percentage of lymphocyte count. No correlation was found between liver injury and age or any coexisting illness (Table 3).

Full table
Effects of liver injury on the prognosis and treatment of COVID-19 pneumonia
As shown in Table 4, in patients with non-severe disease, liver injury was more common in males than females (84.6% vs. 37.5%, P=0.003). Individuals with liver injury presented with higher levels of ALT, AST, and GGT than those without liver injury, regardless of the severity of their disease (all P<0.05). However, no difference in the level of total bilirubin was recorded (P>0.05).
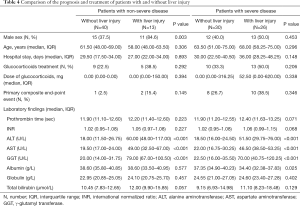
Full table
During hospitalization, in the non-severe patient group, glucocorticoids were used to treat 5 individuals with liver injury and 9 individuals without liver injury (38.5% vs. 22.5%, P=0.292). No significant difference was found between non-severe patients with and without liver injury in terms of glucocorticoid dosage (P=0.913) or hospital stay (P=0.977). Additionally, 2 individuals with liver injury and 1 individual without liver injury underwent noninvasive ventilation (15.4% vs. 2.5%, P=0.145). None of the non-severe patients underwent invasive ventilation, and there were no deaths in patients with and without liver injury.
Among patients with severe disease, no significant difference was observed in the use or dosage of glucocorticoids, hospital stay, or the incidence of primary composite end-point events between those who presented with and without liver injury (all P>0.05). Two individuals with liver injury and 3 individuals without liver injury died of the infection (7.7% vs. 10.0%, P=0.763).
A logistic regression analysis was carried out to assess the possible risk factors for primary composite end-point events (Table 5). The degree of disease severity at admission (OR =7.833, 95% CI, 1.834–31.212, P=0.005) and the presence of any coexisting illness (OR =4.736, 95% CI, 1.305–17.186, P=0.018) were found to be predictable risk factors for primary composite end-point events, whereas liver injury was not (OR =0.549, 95% CI, 0.477–5.156, P=0.459).

Full table
Discussion
In the present study, at admission, 35.8% of patients with COVID-19 pneumonia presented with liver injury, which was more common in patients with severe COVID-19 pneumonia than in those with non-severe disease. However, among patients with severe disease and non-severe disease, no difference was found in the use of mechanical ventilation, mortality, hospital stay, or the use or dosage of glucocorticoids between individuals who presented with and without liver injury. Moreover, liver injury at admission did not show an independent correlation with the occurrence of primary composite end-point events.
Liver injury was reported to be a common clinical manifestation in patients with SARS-CoV infection (14,15), and pathological examinations confirmed the presence of the virus in liver tissue of patients with SARS (8). SARS-CoV-2 has been found to share about 80% of its genetic sequence with SARS-CoV and has the same virus entry receptor, and similar to SARS, liver injury is also common in patients with COVID-19 infection (1,9). More than a third of patients with COVID-19 pneumonia in this investigation presented with liver injury at admission, which manifested mainly as an elevated ALT or AST levels accompanied simultaneously by an increase in GGT levels. Liver injury was found to be more common in patients with severe disease, who had higher levels of AST and GGT. However, there was no significant difference in the levels of total bilirubin or prothrombin time, both of which are considered to be indicators of liver function, between patients with severe and non-severe disease. Serum albumin level, which is an indicator of liver function, was observed to be significantly decreased in patients with severe disease compared to those with non-severe disease. To some extent, serum albumin levels reflect liver synthetic function; however, they may be affected by the nutritional status of the patient. Patients with severe COVID-19 have been reported to be more likely to suffer from malnutrition (1,16). Therefore, in patients with COVID-19 infection, liver injury does not seem to give rise to liver dysfunction. Our results were consistent with the liver pathological findings in patients with COVID-19 infection reported previously (16-18). An autopsy performed on a patient with COVID-19 identified no definite change in the macroscopic appearance of the liver (17). Also, in the liver biopsy specimens of two patients died of COVID-19 pneumonia, moderate microvesicular steatosis, and mild lobular and portal inflammation were observed in the liver tissue of one patient, and mild sinusoidal dilatation and minimal lymphocytic infiltration were observed in the liver tissue of another (16,18). These results indicate that liver injury in patients with COVID-19 is mild, and often does not cause extensive hepatocellular damage, nor does it cause liver dysfunction.
Currently, there are no specific medications available to treat COVID-19 infection, and comprehensive treatments including antiviral treatment, the use of antibiotics, and nutritional support, are recommended (3,11). In China, traditional Chinese medicine is also used to treat COVID-19 infection (11). However, these medications may also cause liver damage, and can exacerbate existing liver injury and even induce liver failure. Therefore, different treatment strategies may be recommended for patients with and without liver injury. Inevitably, the effect of such treatment strategy modifications on the therapeutic efficacy in patients with COVID-19 infection is a concern. Nevertheless, in this study, the incidence of primary composite end-point events, which included the use of mechanical ventilation and mortality during hospitalization, was not higher in individuals with liver injury than in those without liver injury. Additionally, the hospital stay for individuals with liver injury was the same as that for patients without liver injury. All of our results indicate that liver injury at admission does not have a negative impact on the prognosis and treatment of patients with COVID-19 pneumonia with different degrees of severity.
Although the guidelines recommend low doses of glucocorticoids for COVID-19 patients with rapid disease progression (11), glucocorticoid use has been controversial in the treatment of COVID-19 (19). Theoretically, glucocorticoid therapy may play a role in the treatment of COVID-19, as it suppresses lung inflammation. Additionally, Chen et al. reported that patients admitted to the intensive care unit (ICU) were unable to defervesce without glucocorticoid therapy (20). In this study, low-dose glucocorticoid therapy was initiated when a patient’s symptoms became significantly worse or rapid progression of lung lesions was indicated by follow-up chest CT examination. No significant difference was observed in the use or dosage of glucocorticoids between individuals with and without liver injury, regardless of disease severity. Our results also indicate that liver injury at admission does not play a significant role in the deterioration of COVID-19 pneumonia during hospitalization. Furthermore, glucocorticoid therapy itself may suppress liver inflammation, which might explain why liver dysfunction was not observed prior to death in the five patients who died this study (data not shown).
A preliminary study found that cholangiocytes are the major cell type expressing ACE2 in the liver, which indicates that SARS-CoV-2 might mainly act on ACE2-positive cholangiocytes, rather than hepatocytes (21). Most importantly, viral inclusions were not observed in the pathological assessment of liver tissue from a patient who died from COVID-19 infection (18). Therefore, the direct attack on hepatocytes by SARS-CoV-2 was not considered to be the main cause of liver injury. Because all patients in this study were confirmed with COVID-19 infection and were hospitalized in isolation wards to avoid cross infection as much as possible, it was impractical for them to undergo abdominal ultrasonography to determine whether fatty liver or chronic liver disease was present or not, or to undergo liver biopsy to confirm the cause of liver injury at admission. However, only one patient in this study was reported to have chronic hepatitis B. More importantly, liver injury was more common in patients with severe disease and was found to be significantly correlated with the severity of COVID-19 and inflammatory markers at admission. Thus, we speculated that hypoxia and systemic inflammatory response might be two major causes of liver injury at admission in patients with COVID-19. Additionally, because fever is the most common symptom during onset of COVID-19 pneumonia, and patients may have taken antipyretics, such as acetaminophen, to relieve symptoms before admission, drug-induced liver injury should also be considered (1,20).
In conclusion, in this study, more than a third of patients with COVID-19 pneumonia presented with liver injury at admission, and this was more common in individuals with severe disease. However, liver injury was mild in most of the patients and had no negative impact on the prognosis and treatment of COVID-19 pneumonia during hospitalization.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4850).
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4850
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-4850
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4850). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of People’s Hospital of Wuhan University (NO. WDRY-2020-K048) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. [Crossref] [PubMed]
- Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565-74. [Crossref] [PubMed]
- Organization World Health. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270-3. [Crossref] [PubMed]
- Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020;63:457-60. [Crossref] [PubMed]
- Eurosurveillance Editorial Team. Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Euro Surveill 2020;25:200131e.
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available online: https://coronavirus.jhu.edu/map.html
- Chau TN, Lee KC, Yao H, et al. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology 2004;39:302-10. [Crossref] [PubMed]
- Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 2020;5:428-30. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. Available online: https://www.chinadaily.com.cn/pdf/2020/1.Clinical.Protocols.for.the.Diagnosis.and.Treatment.of.COVID-19.V7.pdf
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Disease Society of America. Am J Respir Crit Care Med 2019;200:e45-67. [Crossref] [PubMed]
- Gao HN, Lu HZ, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 2013;368:2277-85. [Crossref] [PubMed]
- Humar A, McGilvray I, Phillips MJ, et al. Severe acute respiratory syndrome and the liver. Hepatology 2004;39:291-4. [Crossref] [PubMed]
- Chan HL, Leung WK, To KF, et al. Retrospective analysis of liver function derangement in severe acute respiratory syndrome. Am J Med 2004;116:566-7. [Crossref] [PubMed]
- Zhang Y, Zheng L, Liu L, et al. Liver injury in COVID-19 patients: a retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver Int 2020;40:2095-103. [Crossref] [PubMed]
- Available online: http://www.fyxzz.cn/CN/abstract/abstract23213.shtml
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [Crossref] [PubMed]
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020;395:473-5. [Crossref] [PubMed]
- Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect 2020;80:e1-6. [Crossref] [PubMed]
- Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv 2020. Available online: https://doi.org/ [Crossref]


