
The role of primary tumor SUVmax in the diagnosis of invasion depth: a step toward clinical T2N0 esophageal cancer
Introduction
Esophageal cancer is one of the most common malignancies worldwide, with a dismal prognosis (1). Histologically, esophageal cancer includes two major subtypes: esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) (2). In recent decades, much attention has been paid towards improving patients’ long-term survival with esophageal cancer. Nowadays, induction therapy (chemotherapy or chemoradiotherapy), followed by surgery, can provide a survival benefit for locally advanced esophageal cancer (3-6). Local therapy (endoscopic or surgical resection) is usually applied for early-stage esophageal cancer, but this remains the subject of much debate.
This debate is derived from the inaccuracy of esophageal cancer’s clinical staging, which is particularly evident in clinical T2N0 (cT2N0). Crabtree et al. found that 34%, 25.3%, and 0.6% of cT2N0 were pathological T0–1 (pT0–1), pT3, and pT4, respectively (7). The Esophageal Cancer Study Group found that 44.5%, 31%, and 1% of cT2N0 were pT0–1, pT3, and pT4, respectively (8). Other groups have reported that more than 50% of cT2 esophageal cancers were pT1 after surgery, even when staged by experienced clinicians (9,10). Consequently, there is much debate surrounding the optimal treatment for cT2N0 esophageal cancer, namely, between primary surgery or induction therapy (11-13).
The current pathological T staging is based on invasion depth. Thus, an accurate clinical T staging demands a high resolution of the esophagus (14), especially for flat tumors and diffused infiltration (15). The fact that most migration of the cT2 category lies in cT2 to pT1 indicates that the common modalities cannot accurately discriminate whether or not the muscularis propria is invaded. Thus, one natural question is whether the metabolic activity may play a role in this process.
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) with computed tomography (CT) is included as part of the initial workup (3-5), but it is usually used to detect distant metastasis (16). Recent studies have found that the maximal standard uptake value (SUVmax) of the primary tumors was associated with pathological features (17-26). We, therefore, hypothesized that metabolic activity would determine whether or not the muscularis propria was invaded. We investigated the potential association between the primary tumor SUVmax and pathological features and overall survival. Furthermore, we attempted to construct certain models to discriminate invasion depth. We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4430).
Methods
Patients
From January 2015 to December 2017, 234 consecutive patients underwent baseline 18F-FDG PET/CT, followed by primary esophagectomy in Zhongshan Hospital Fudan University. Of these patients, 67 were clinically staged as cN+, while 2 had received endoscopic dissection (ESD) before 18F-FDG PET/CT, and 25 were histologically confirmed as not ESCC. Eventually, 140 patients were enrolled in this retrospective study. This study had been approved by the Institutional Review Board of Zhongshan Hospital Fudan University (HGBB-202006001) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The informed consent was waived as the nature of retrospective studies.
As the reference standard, the pathological staging was based on the TNM Staging System (8th edition, 2017) (14). Tumors that invaded the lamina propria or muscularis mucosae, the submucosa, the muscularis propria, the adventitia, and the adjacent structures were defined as pT1a, pT1b, pT2, pT3, and pT4, respectively. All of the patients had discernible pT categories in this study.
Follow-up
Patients were asked to receive physical examination, tumor markers testing, thoracic CT, and cervical and abdominal ultrasonography in the outpatient clinic. For patients with particular signs or symptoms, additional examinations were conducted. A combination of clinical service records and phone calls were used to determine each patient’s status as of March 2020. In this study, overall survival was defined as the interval between the date of esophagectomy and the date of death or the last follow-up date.
PET/CT protocol and interpretation
According to the routine protocol of 18F-FDG PET-CT at our institution, the patients fasted for at least 6 hours, and the serum glucose levels were required to be lower than 11.0 mmol/L before imaging. 18F-FDG PET/CT scans were performed on a hybrid GE Discovery VCT 64 PET/CT scanner (General Electric, Milwaukee, WI, USA) from the proximal thigh to the skull base. Metabolic images were obtained approximately 60 minutes after intravenous administration of 3.7 to 5.6 MBq of FDG per kilogram of body weight. PET images were acquired for 2 minutes per bed position. The CT scanning was performed on the same scanner without contrast administration (200 mA, 120 kV, matrix 512×512, 0.8 s per rotation). The SUV was normalized to body weight as a determinate index. All PET-CT scans were performed within 1 month before surgery.
Statistical analyses
Continuous data were presented as mean with standard deviation (SD) or median with interquartile ranges (IQRs). Categorical data were presented as numbers with a proportion (%). Both parametric and non-parametric tests were used, including the Chi-square test and the Kruskal-Wallis test. The association between baseline characteristics and pathological findings was determined by univariate and multivariate logistic regression analysis. The variables with P<0.10 in the univariate regression were included in the multivariate regression. The independent factors were included in the predictive model. The receiver operator curve (ROC) with Youden’s index (27) was used to determine the predictive performance and identify the optimal cutoff of continuous parameters. The area under the curve (AUC) with 95% confidence interval (CI) was used to measure the discriminating performance. The Kaplan-Meier method was used to generate survival curves, and the log-rank test was used to evaluate survival differences. All patients included in the survival analysis were followed up for at least 3 months after surgery or until death.
Statistical analyses were performed using SPSS version 24 (SPSS Inc., Chicago, IL, USA) and R software version 1 3.5.1 (Packages: survival and survminer). P<0.05 was considered statistically significant.
Results
Preoperative characteristics of the patients
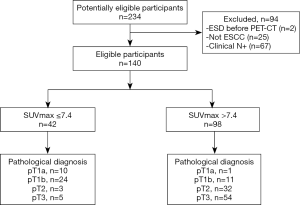
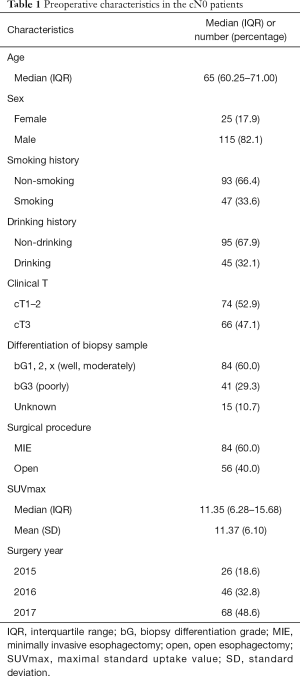
As shown in Figure 1 and Table 1, a total of 140 cN0 patients were enrolled in this study, including 25 females and 115 males, with a median age of 65 (IQR: 60.25–71.00). Of the 140 patients, 74 (52.9%) were clinically staged as cT1–2, and 66 (47.1%) were clinically staged as cT3. Based on the endoscopic biopsy, 41 (29.3%) tumors were poorly differentiated, 84 (60.0%) tumors were well or moderately differentiated, while 15 (10.7%) tumors had no grading information. The median and mean SUVmax were 11.35 (IQR: 6.28–15.68) and 11.37 (SD: 6.10), respectively.
Full table
Postoperative characteristics and their association with SUVmax
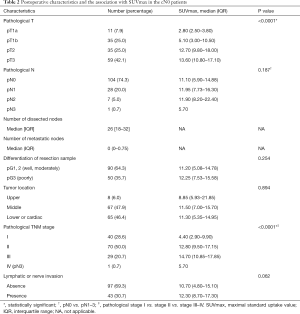
For all patients, surgical resection with the pathological examination was conducted within 1 month after the completion of PET-CT, and no cancer-related treatment was administered during this interval. Histologically, 11 (7.9%), 35 (25.0%), 35 (25.0%), and 59 (42.1%) tumors were confirmed as pT1a (mucosa), pT1b (submucosa), pT2 (muscularis propria), and pT3 (adventitia), respectively, while 36 (25.7%) were staged as pN+. There was a significant correlation between invasion depth and node metastasis (P=0.017, Table S1). Ultimately, 110 (78.6%) were in pathological stage I–II, and 30 (21.4%) were in pathological stage III–IV. Lymphatic or nerve invasion (LNI) was present in 43 (30.7%) patients.
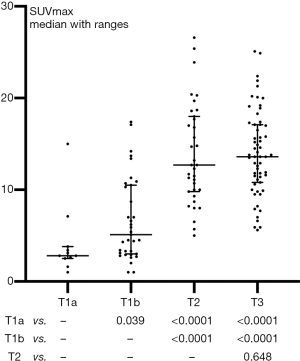
As shown in Figure 2, the primary tumor SUVmax differed significantly between any paired pT categories (all P<0.05), but not between pT2 vs. pT3 (P=0.648). As shown in Table 2, although SUVmax also differed significantly between pathological stage categories (pStage I vs. II vs. III–IV, P<0.0001), we found it was not significantly different in pN categories (pN0 vs. pN+, P=0.187), pG categories (well or moderately vs. poorly, P=0.254), and LNI categories (absence vs. presence, P=0.062).
Full table
Baseline risk factors for invasion depth
According to the univariate and multivariate logistic regression analysis, age (≤65 vs. >65), biopsy differentiation (well or moderately vs. poorly vs. unknown), and SUVmax (continuous) were identified as the baseline risk factors for the invasion of the muscularis propria (Table 3).
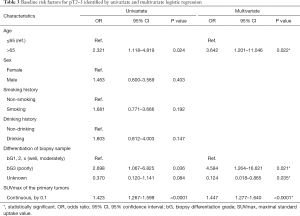
Full table
Of these factors, the ROC analysis suggested that SUVmax (continuous) demonstrated good discriminative performance for both pT2 vs. pT1 (n=81, AUC: 0.866, 95% CI: 0.790 to 0.942, P<0.0001, Figure 3A) and for pT2–3 vs. pT1 (n=140, AUC: 0.877, 95% CI: 0.811 to 0.943, P<0.0001, Figure 3B). Youden’s index was used to determine the cutoff as 7.4 for SUVmax (categorized) in all patients to fit clinical applications. The discriminating outcomes are presented in Figure 1.
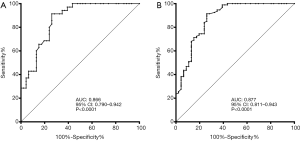
The discriminating model for pT2–3
According to the identification of baseline risk factors, the age categories (≤65 vs. >65), biopsy differentiation categories (well or moderately vs. poorly vs. unknown), and the primary tumor SUVmax categories (≤7.4 vs. >7.4) were included in the logistic regression analysis (enter method) to construct a novel model. As shown in Table 4, this model demonstrated good performance: accuracy 87.1%, sensitivity 93.6%, specificity 73.9%, positive predictive value (PPV) 88.0%, and negative predictive value (NPV) 85.0%. The Hosmer-Lemeshow test indicated a good calibration (Chi-square 2.289, P=0.891, Figure 4A), and the ROC analysis indicated high discrimination (AUC: 0.887, 95% CI: 0.822 to 0.951, P<0.0001, Figure 4B) of the new model. The distribution of pT2–3 vs. pT1 in the stratification of the model is shown in Table S2.
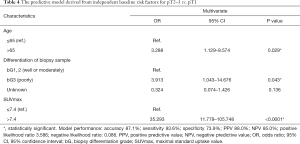
Full table
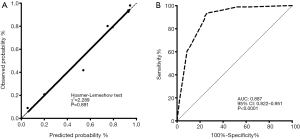
Prognostic value of the baseline risk factors
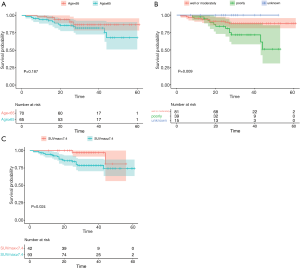
We then investigated whether the baseline risk factors were associated with overall survival. After excluding 5 patients lost to follow-up, 135 patients were included in the survival analysis. The median follow-up period was 35 months, and the 3-year overall survival rate was 84.3% (78.0% to 91.1%). We found that age (P=0.187, Figure 5A) showed no significant difference, while biopsy differentiation (P=0.009, Figure 5B) and primary tumor SUVmax (P=0.024, Figure 5C) showed significant differences in overall survival.
Discussion
The heated debate regarding cT2N0 esophageal cancer ultimately stems from the current clinical staging model’s considerable inaccuracy. Pech et al. reported that the accuracy of cT1, cT2, and cT3 staged by endoscopic ultrasound (EUS) was 92%, 37%, and 68%, respectively (28). Dhupar et al. reported that the accuracy of cT1a, cT1b, cT2, and cT3 staged by EUS was 56%, 58%, 10%, and 70%, respectively (29). Apart from EUS, CT, which relies on wall thickness, cannot provide adequate information (30-32). Therefore, further study in this field would be on shaky ground as long as this problem remains unsolved. Because most of the cT2 category’s migration lies in cT2 to pT1, finding effective methods to discriminate muscularis propria invasion is of great importance.
Consistent with previous studies (17-26), an increasing trend of primary tumor SUVmax was observed in the advanced pT categories. In this circumstance, the core question was whether this increasing trend could be translated to discriminate invasion depth prospectively. Huang et al. previously reported a positive result in a small study (n=45), where it showed great discriminative performance for pT categories (≥ pT1, AUC: 1.00; ≥ pT2, AUC: 0.88; and ≥ pT3, AUC: 0.95) (17). However, in our study, we found SUVmax differed significantly in paired pT categories but not pT2 vs. pT3. Consequently, we found SUVmax (continuous) had good discrimination for pT2–3 vs. pT1, and pT2 vs. pT1, but not for pT3 vs. pT1–2 (AUC: 0.730, 95% CI: 0.648 to 0.812). The discrepancy between studies might be attributed to institutional variations and selection bias. However, in general, the current published studies have similar outcomes regarding the difference in metabolic uptake between pT1 and their counterparts (9,17-19,31).
Although SUVmax (continuous) demonstrated good performance, we determined its optimal cutoff to fit clinical applications. The subsequent logistic regression model comprised of SUVmax (categorized), age, and biopsy differentiation achieved better discriminative performance. To our knowledge, this is the first comprehensive baseline model aimed at discriminating invasion depth. Furthermore, the model elements are more objective and less variable compared to EUS, which is technically demanding. Although endoscopic resection or dissection is useful to identify the mucosal or submucosal status, it cannot evaluate muscularis propria invasion due to the risk of severe perforation and delayed treatment. In our opinion, this novel model warrants consideration in future clinical trials.
With regards to the other pathological features, however, the published findings are not consistent. Some studies have reported that primary tumor SUVmax was correlated significantly with node metastasis, tumor differentiation, lymphatic invasion, and perineural invasion (17-20). However, other groups have reported contradictory outcomes (24-26). In our study, although invasion depth (pT2–3 vs. pT1) was correlated with SUVmax (Spearman, P<0.0001), and with pN, pG, and LNI categories (Spearman, P=0.016, P=0.001, P<0.001, respectively), SUVmax was not significantly correlated with pN, pG, and LNI categories. However, regarding the correlation between SUVmax and pathological staging (Spearman, P=0.008), we believed that it was the correlation between SUVmax and invasion depth (pT2–3 vs. pT1) that worked as a bridge.
To our study, the sharp question is what is the foundation of this bridge, considering the limited spatial resolution of 18F-FDG PET. This is currently not well understood. However, in a previous study in small-sized lung adenocarcinoma, evidence has demonstrated that SUVmax differed significantly between different histological subtypes and correlated with node metastasis (33). Although several studies have reported the differences of SUVmax in invasion depth (9,17-19,31), the biological mechanism remains to be elucidated. We posit that in early-stage cancer, the metabolic activity can provide certain information ahead of the visualization of spatial changes on current imaging modalities.
Previous studies have found that the current modalities are inadequate to determine the cT2 category (9,10,28,29,34,35). Thus, EUS is not mandatory at our institution despite the guideline recommendations (3-5). It is often absent in real-world studies with large cohorts (8), including clinical trials (36). This is one limitation of our study. However, the satisfactory performance of SUVmax (continuous) and our newly proposed model highlight a promising role for metabolic activity in discriminating invasion depth.
There were some other limitations in our study. Firstly, this was a retrospective study with inherent selection bias. However, all patients received baseline PET-CT in our institution, which ensured a consistent protocol and interpretation. Secondly, some preoperative characteristics, such as endoscopic tumor diameter or length, were not included. However, the endoscopic measurement can have some errors in early-stage esophageal cancer. Moreover, in our study, 15 patients had no grading information, which would have certain effects on the model. Finally, although this model showed a satisfactory performance, it was not able to further discriminate against the invasion of muscularis propria (pT2) and adventitia (pT3), though cT3 tumors can often be identified by current modalities (37). This study is, therefore, a step toward addressing the heated debate on cT2N0 esophageal cancer. A prospective trial led by a multidisciplinary team is necessary to validate the findings and guide clinical practice.
Conclusions
Our novel baseline model comprised of age, biopsy differentiation grades, and primary tumor SUVmax provides a considerable discriminative performance in determining whether or not the muscularis propria is invaded. Further studies are necessary to validate the findings and guide clinical practice for cT2N0 esophageal cancer.
Acknowledgments
We sincerely acknowledge Zhengyang Hu for the help in statistical analyses, and C. Betlazar-Maseh and J. Chapnick from AME Editing Service for their help in language editing.
Funding: This study was supported by The Institutional Funding of Zhongshan Hospital Fudan University (No. 2019ZSFZ16, No. 2016ZSLC15). The funding had no influence on the design and interpretation of the study.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4430
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4430
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4430). Dr. HW serves as an unpaid editorial board member of Annals of Translational Medicine from Jul 2019 to Jun 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study had been approved by the Institutional Review Board of Zhongshan Hospital Fudan University (HGBB-202006001) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The informed consent was waived as the nature of retrospective studies.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Napier KJ, Scheerer M, Misra S. Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014;6:112-20. [Crossref] [PubMed]
- Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2012 edited by the Japan Esophageal Society. Esophagus 2015;12:1-30.
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50-7. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:855-83. [Crossref] [PubMed]
- D'Journo XB, Thomas PA. Current management of esophageal cancer. J Thorac Dis 2014;6 Suppl 2:S253-64. [PubMed]
- Crabtree TD, Kosinski AS, Puri V, et al. Evaluation of the reliability of clinical staging of T2 N0 esophageal cancer: a review of the Society of Thoracic Surgeons database. Ann Thorac Surg 2013;96:382-90. [Crossref] [PubMed]
- Esophageal Cancer Study Group Participating Centers. Predictors of staging accuracy, pathologic nodal involvement, and overall survival for cT2N0 carcinoma of the esophagus. J Thorac Cardiovasc Surg 2018. [Epub ahead of print]. [PubMed]
- Nelson DB, Mitchell KG, Weston BR, et al. Should endoscopic mucosal resection be attempted for cT2N0 esophageal cancer? Dis Esophagus 2019;32:1-6. [Crossref] [PubMed]
- Luu C, Amaral M, Klapman J, et al. Endoscopic ultrasound staging for early esophageal cancer: are we denying patients neoadjuvant chemo-radiation? World J Gastroenterol 2017;23:8193-9. [Crossref] [PubMed]
- Semenkovich TR, Panni RZ, Hudson JL, et al. Comparative effectiveness of upfront esophagectomy versus induction chemoradiation in clinical stage T2N0 esophageal cancer: a decision analysis. J Thorac Cardiovasc Surg 2018;155:2221-30.e1. [Crossref] [PubMed]
- Samson P, Puri V, Robinson C, et al. Clinical T2N0 esophageal cancer: identifying pretreatment characteristics associated with pathologic upstaging and the potential role for induction therapy. Ann Thorac Surg 2016;101:2102-11. [Crossref] [PubMed]
- Speicher PJ, Ganapathi AM, Englum BR, et al. Induction therapy does not improve survival for clinical stage T2N0 esophageal cancer. J Thorac Oncol 2014;9:1195-201. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol 2017;12:36-42.
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus 2017;14:1-36.
- Kim TJ, Kim HY, Lee KW, et al. Multimodality assessment of esophageal cancer: preoperative staging and monitoring of response to therapy. Radiographics 2009;29:403-21. [Crossref] [PubMed]
- Huang YC, Lu HI, Huang SC, et al. FDG PET using SUVmax for preoperative T-staging of esophageal squamous cell carcinoma with and without neoadjuvant chemoradiotherapy. BMC Med Imaging 2017;17:1. [Crossref] [PubMed]
- Kato H, Kuwano H, Nakajima M, et al. Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer 2002;94:921-8. [Crossref] [PubMed]
- Lim CH, Park YJ, Shin M, et al. Tumor SUVs on 18F-FDG PET/CT and aggressive pathological features in esophageal squamous cell carcinoma. Clin Nucl Med 2020;45:e128-33. [Crossref] [PubMed]
- Miyawaki Y, Sato H, Fujiwara N, et al. Association of the primary tumor's SUVmax with survival after surgery for clinical stage IA esophageal cancer: a single-center retrospective study. Int J Clin Oncol 2020;25:561-9. [Crossref] [PubMed]
- Kita Y, Okumura H, Uchikado Y, et al. Clinical significance of 18F-fluorodeoxyglucose positron emission tomography in superficial esophageal squamous cell carcinoma. Ann Surg Oncol 2013;20:1646-52. [Crossref] [PubMed]
- Sun G, Tian J, Gorospe EC, et al. Utility of baseline positron emission tomography with computed tomography for predicting endoscopic resectability and survival outcomes in patients with early esophageal adenocarcinoma. J Gastroenterol Hepatol 2013;28:975-81. [Crossref] [PubMed]
- Kaida H, Kawahara A, Hayakawa M, et al. The difference in relationship between 18F-FDG uptake and clinicopathological factors on thyroid, esophageal, and lung cancers. Nucl Med Commun 2014;35:36-43. [Crossref] [PubMed]
- Sun M, Li B, Fu Z, et al. Relationship between (18)F-fluorodeoxyglucose uptake in primary lesions and clinicopathological characteristics of esophageal squamous cell carcinoma patients. Exp Ther Med 2013;5:170-4. [Crossref] [PubMed]
- Jiang W, Yang J, Lin X, et al. 18F-FDG PET-CT metabolic findings can predict the short-term curative effects in esophageal cancer. Int J Clin Exp Pathol 2019;12:4130-6. [PubMed]
- Shimizu D, Yuasa N, Miyake H, et al. Clinical significance of SUVmax on preoperative 18F-fluorodeoxyglucose positron emission tomography in patients who underwent R0-esophagectomy for esophageal cancer. Nagoya J Med Sci 2018;80:401-9. [PubMed]
- Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J 2005;47:458-72. [Crossref] [PubMed]
- Pech O, Günter E, Dusemund F, et al. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy 2010;42:456-61. [Crossref] [PubMed]
- Dhupar R, Rice RD, Correa AM, et al. Endoscopic ultrasound estimates for tumor depth at the gastroesophageal junction are inaccurate: implications for the liberal use of endoscopic resection. Ann Thorac Surg 2015;100:1812-6. [Crossref] [PubMed]
- Bunting D, Bracey T, Fox B, et al. Loco-regional staging accuracy in oesophageal cancer-How good are we in the modern era? Eur J Radiol 2017;97:71-5. [Crossref] [PubMed]
- Jeong DY, Kim MY, Lee KS, et al. Surgically resected T1- and T2-stage esophageal squamous cell carcinoma: T and N staging performance of EUS and PET/CT. Cancer Med 2018;7:3561-70. [Crossref] [PubMed]
- Lowe VJ, Booya F, Fletcher JG, et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422-30. [Crossref] [PubMed]
- Nakamura H, Saji H, Shinmyo T, et al. Close association of IASLC/ATS/ERS lung adenocarcinoma subtypes with glucose-uptake in positron emission tomography. Lung Cancer 2015;87:28-33. [Crossref] [PubMed]
- Hong SJ, Kim TJ, Nam KB, et al. New TNM staging system for esophageal cancer: what chest radiologists need to know. Radiographics 2014;34:1722-40. [Crossref] [PubMed]
- Bergeron EJ, Lin J, Chang AC, et al. Endoscopic ultrasound is inadequate to determine which T1/T2 esophageal tumors are candidates for endoluminal therapies. J Thorac Cardiovasc Surg 2014;147:765-71; discussion 771-3. [Crossref] [PubMed]
- Klevebro F, Alexandersson von Döbeln G, Wang N, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol 2016;27:660-7. [Crossref] [PubMed]
- Chao YK, Ku HY, Chen CY, et al. Induction therapy before surgery improves survival in patients with clinical T3N0 esophageal cancer: a nationwide study in Taiwan. Dis Esophagus 2017;30:1-7. [Crossref] [PubMed]


