
Acute vasoreactivity testing predicts outcome of idiopathic pulmonary arterial hypertension patients with a negative acute response
Introduction
Idiopathic pulmonary arterial hypertension (IPAH) is a progressive, devastating, and incurable disease characterized by structural changes to the small pulmonary arteries, which lead to increased pulmonary vascular resistance (PVR) ultimately resulting in right heart failure and death (1,2). Right heart catheterization (RHC) remains the gold standard procedure to confirm the diagnosis of pulmonary hypertension (PH) (3). Acute vasoreactivity testing (AVT) is an additional examination to identify of patients suitable for high-dose calcium channel blocker (CCB) treatment is recommended only for patients with idiopathic PAH (IPAH), heritable PAH) or drug-induced PAH (4). AVT should be completed during the first RHC. A positive acute response is defined as a reduction of the mean pulmonary arterial pressure (mPAP) ≥10 mmHg and the absolute value of mPAP ≤40 mmHg with an increased or unchanged cardiac output (CO) (4). Although this positive acute response is found in less than 10% of patients with such a deadly progressive disease, it has important clinical significance in both the diagnosis and treatment of these patients (5).
In 2015, Leuchte et al. (6) reported that changes in PVR (Δ PVR) during AVT were of prognostic relevance for 66 patients with IPAH who presented with a negative acute response. They found that post-CO, post-cardiac index (CI), and post- mixed venous oxygen saturation (SvO2) parameters were increased, while post-mPAP and post-PVR parameters were decreased after AVT, but the prognostic value of these parameters was not further explored. Recently, our center conducted two studies on evaluating the prognostic value of pre-AVT, post-AVT and changed AVT (ΔAVT) parameters in patients with chronic thromboembolic pulmonary hypertension (CTEPH) (7,8). One study found that pre-mixed venous oxygen saturation (pre-SvO2), post-PVR, and ΔPVR/PVR could be used as independent parameters to predict outcomes of patients with CTEPH (7). The other study demonstrated that a mean right atrial pressure (mRAP) ≥8.0 mmHg and SvO2 ≤61.8% both pre-AVT and post-AVT were independent predictors of event-free survival for females with CTEPH, whereas ΔSvO2 ≤0.6 was an independent predictor of event-free survival for males with CTEPH (8). These suggest that different hemodynamic parameters during the AVT have discrete prognostic values for both sexes (8).
Based on the above findings, the present study aimed to investigate whether pre-AVT, post-AVT, and ΔAVT parameters have prognostic value for IPAH patients with a negative acute response, and whether the sex difference in AVT parameters provides more hemodynamic information for patients with IPAH.
We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/atm-20-7339).
Methods
Study design and participants
A total of 487 patients (171 males and 316 females) with incident IPAH were enrolled at Shanghai Pulmonary Hospital between February 2009 and September 2019. A diagnosis of IPAH was defined as mPAP ≥25 mmHg, pulmonary capillary wedge pressure (PCWP) ≤15 mmHg, and PVR >3 Wood units as measured by RHC in accordance with the European Society of Cardiology (ESC) guidelines (3,9). Exclusion criteria were as follows: (I) PH associated with anorexigens, connective tissue diseases, congenital heart diseases, portal hypertension, or HIV infection; (II) other chronic respiratory diseases; (III) patients with a negative acute response (9) during RHC; (IV) patients with acute or chronic illnesses that might affect hormonal metabolism (i.e., acute or chronic infections, chronic autoimmune diseases, and previously established primary endocrine disorders) and patients receiving any treatment with hormones (anabolic steroids, thyroid hormones, and corticosteroids) or drugs that significantly inhibit hormone production, either at the time of the study or in the past (4,10,11).
This study complied with the Declaration of Helsinki (as revised in 2013) and was approved by the Medical Ethics Committee of Shanghai Pulmonary Hospital. Written informed consent was obtained from either the patients or their next of kin.
RHC and AVT assessment
None of the patients were receiving any PAH therapies at the time of the RHC. An 8F introducer sheath (St Jude Medical Inc., MN, USA) was placed in the right internal jugular vein or the right subclavian vein, and a quadric-lumen 7F Swan-Ganz catheter (Edwards Lifesciences, Irvine, CA, USA) was inserted into the pulmonary artery. The correct positioning of the catheter was verified by chest fluoroscopy. The mPAP, mRAP, and PCWP parameters were measured at baseline and after vasodilator administration. The CO was measured in triplicate using the thermodilution technique (Edwards Lifesciences) with an ice-cold isotonic sodium chloride solution. PVR was calculated as (mPAP − PCWP)/CO. Heart rate (HR), electrocardiogram, systemic arterial pressure, and oxygen saturation were measured continuously. In addition, arterial blood gases and SvO2 were measured (ABL 555; Radiometer, Copenhagen, Denmark) (12).
After a stable baseline period of at least 20 minutes, each patient was asked to inhale 5 mg of iloprost (Ventavis, BayerVital, Germany) via a mouthpiece for a duration of approximately 15 minutes. The hemodynamic parameters were measured immediately after the iloprost inhalation ended and 15 minutes after the end of the aerosolization period when the maximal response was recorded.
Targeted therapies
Three well-known pathways contribute to the pathogenesis of PAH: the endothelin, NO and prostacyclin pathways. Based on the three pathways, more than ten targeted drugs have been applied in clinical practice, including endothelin receptor antagonists (ambrisentan, bosentan and macitentan), phosphodiesterase type 5 inhibitors and guanylate cyclase stimulators (sildenafil, tadalafil and riociguat) and prostacyclin analogues and prostacyclin receptor agonists (Berapros and epoprostenol). In our center, physicians will decide whether to use a single target drug or a combination therapy for IPAH patients with negative vascular response test.
Follow-up of patients
Follow-up intervals were determined by physicians based on the individual patient’s healthcare needs. Patients with IPAH were encouraged to visit our outpatient department or to phone us every 3–6 months according to the ESC guidelines. The outcome was all-cause mortality. Survival rate was estimated from the date of diagnosis to 5th January, 2020.
Statistical analysis
Results were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) for continuous variables and as percentages (%) for categorical variables. Comparison of the clinical characteristics between patients was performed using the Chi-square test for categorical data and the Student’s t test or Mann-Whitney U test for continuous data. The impact of parameters on prognosis was evaluated using univariate and multivariate Cox proportional hazards analyses. Age and body surface area (BSA) were forced into models to adjust the multivariate analysis. A receiver operating characteristic (ROC) analysis was used to determine the area under the curve (AUC) for continuous variables identified from the multivariate regression analysis, and optimal cut points were determined by the value which results in the maximum sum of sensitivity and specificity. Survival curves were derived using the Kaplan-Meier method and were compared using the log-rank test.
All statistical analyses were performed using SPSS (Statistical Package for the Social Sciences, Chicago, IL, USA) software version 25.0 and GraphPad Prism (San Diego, CA, USA) version 8.0.
Results
Baseline characteristics
Clinical characteristics, results of laboratory tests, parameters of pre-AVT, and targeted therapies are shown in Table 1. The mean ages of 171 males and 316 females with IPAH were 41.08±20.60 and 36.68±15.49 years, respectively. Both age and BSA were significantly higher in males than in females. No significant differences were found between males and females in the 6-minute walking distance (6MWD) test, the World Health Organization Functional Classification (WHO-FC) system, and the levels of N-terminal pro-brain natriuretic peptide (NT-proBNP). There was no significant difference in the use of medications between male and female patients.

Full table
Comparison of pre-AVT hemodynamic parameters
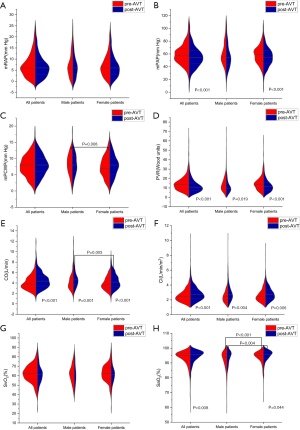
The pre-mean PCWP (pre-mPCWP) and pre-artery oxygen saturation (pre-SaO2) indices were higher in males than in females. The pre-mRAP, pre-mPAP, pre-PVR, pre-CO, pre-cardiac index (pre-CI), and pre-SvO2 parameters did not show any significant difference between male and female patients (Table 1). The levels of pre-mRAP, pre-mPAP, pre-mPCWP, and pre-PVR were significantly higher in non-survivors than in survivors for all patients, and pre-CO, pre-CI, and pre-SvO2, indices were significantly lower in non-survivors than in survivors (Figure 1). We also compared sex differences in pre-AVT parameters between survivors and non-survivors. Interestingly, the pre-mPCWP level was higher in male non-survivors than in female non-survivors (Figure 1C). However, pre-CO was higher and pre-SaO2 was lower in male survivors than in female survivors (Figure 1E,H). Pre- SaO2 was also lower in male non-survivors than in female non-survivors (Figure 1H).
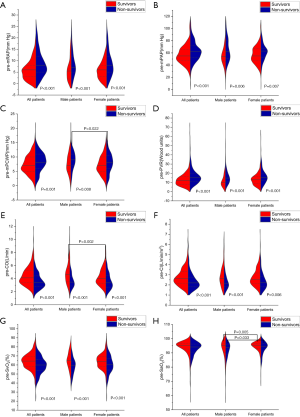
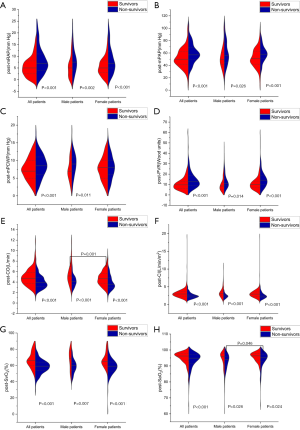
Comparison of post-AVT hemodynamic parameters
The parameters of post-AVT were measured 15 minutes after inhaling the iloprost aerosol, and it was found that post-CO was higher and post-SaO2 was lower in male patients than in female patients. However, there was no significant difference in other parameters of post-AVT between male and female patients (Table 2). The levels of post-mRAP, post-mPAP, and post-PVR were significantly higher in non-survivors, and levels of post-CO, post-CI, post-SvO2, and post-SaO2 were significantly lower in non-survivors than in survivors (Figure 2). The post-mPCWP level was also significantly increased in all non-survivors and male non-survivors (Figure 2C). Differences in sex were investigated in the post-AVT parameters between survivors and non-survivors and we found that the post- SaO2 level was lower in male non-survivors than in female non-survivors (Figure 2H).

Full table
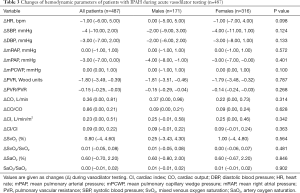
Comparison of ΔAVT hemodynamic parameters
The parameters of ΔAVT of male patients were similar to those of female patients (Table 3). And the comparison of ΔAVT between survivors and non-survivors is show in Figure 3. In females, the absolute changes of ΔmPAP and ΔPVR were significantly higher in survivors than in non-survivors (Figure 3B,D) whereas no significant difference was observed in male patients. And ΔPVR was higher in male survivors than in female survivors (Figure 3D). Male survivors demonstrated lower ΔCO than female survivors (Figure 3E). We further discovered the importance of ΔPVR/PVR, which is higher among all non-survivors and female non-survivors than among survivors, For survivors, the absolute changes of ΔPVR/PVR in male survivors is higher than that in female survivors (Figure 3I). Furthermore, ΔCI/CI was higher in male non-survivors than in female non-survivors (Figure 3J).
Full table
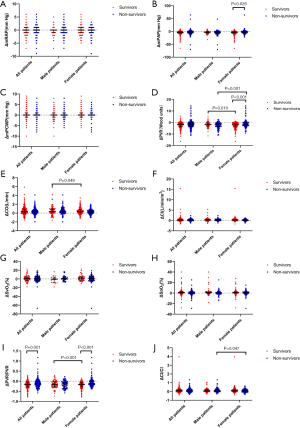
Changes of hemodynamic parameters pre- and post-AVT
The comparison of parameters pre- and post-AVT for patients is shown in Figure 4. Post-mPAP and post-PVR levels were lower than pre-mPAP and pre-PVR levels, respectively, for all patients and female patients, while post-CO, post-CI, and post-SaO2 levels were higher than pre-CO, pre-CI, and pre-SaO2 levels. Post-PVR was lower than the pre-PVR level, and the post-CO and post-CI levels were higher than the pre-CO and pre-CI levels, respectively, for male patients.
Independent prognostic parameters in hemodynamics
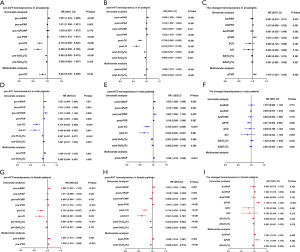
The results of the univariate and multivariate analyses in hemodynamic parameters are shown in Figure 5. In general, pre-CO, post-mPAP, post-SvO2, and ΔPVR levels were associated with patients’ prognosis (Figure 5A,B,C). In multivariate analysis, pre-PVR, pre-SvO2, and post-mPAP levels were independent predictors for survival of male patients (Figure 5D,E), whereas the pre-mRAP, pre-PVR, post-PVR, post-SvO2, ΔmPAP, ΔPVR, and ΔSvO2 parameters were independent predictors for survival of female patients (Figure 5G,H,I).
ROC analysis in patients with IPAH
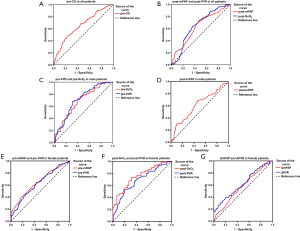
ROC analysis was used to evaluate the ability of parameters that had a significant correlation with survival in the multivariate analysis to predict survival. Results are presented in Table 4 and Figure 6. In general, pre-CO =3.25 L/min showed a sensitivity of 55.9% and a specificity of 80.0% in predicting survival (Figure 6A), while post-mRAP =53 mmHg showed a sensitivity of 68.1% and a specificity of 56.9% in predicting survival. In addition, the cut-off value for post-SvO2 was 63.4% with a sensitivity of 74.8% and a specificity of 31.1% (Figure 6B). In regard to the parameters for male non-survivors, the areas under the curve of pre-PVR, pre-SvO2, and post-mPAP were 0.706, 0.670, and 0.608, respectively (Figure 6C,D). The cut-off values were 12.47 Wood units, 64%, and 51 mmHg, respectively. Pre-mRAP, pre-PVR, post-PVR, post-SvO2, ΔmPAP, and ΔPVR parameters showed a significant correlation with prognosis, and the initial cut-off values for death prediction were 9.0 mmHg, 13.65 Wood units, 13.87 Wood units, 63%, −4 mmHg, and −0.25 Wood units, respectively (Figure 6E,F,G). However, ΔSvO2 did not show significant correlation with survival in the ROC analysis (P=0.354).

Full table
Prognostic implication of AVT in patients with IPAH
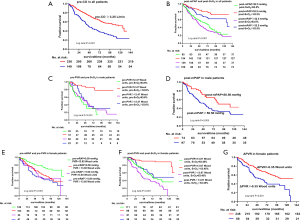
During the follow-up period (median 46.20, IQR, 18.90–85.43 months), 214 (n=487; 43.94%) patients died. The cut-off values of the included parameters for the fraction of survival based on the Kaplan-Meier curve analysis are shown in Figure 7. In general, patients with pre-CO ≥3.25 L/min, post-mPAP <53 mmHg, or post-SvO2 ≥63% had a better prognosis (Figure 7A,B). Male patients with pre-PVR <12.47 Wood units, pre-SvO2 ≥64%, and post-mPAP <51 mmHg had a better prognosis than other male patients (Figure 7C,D). Female patients with pre-mRAP <9.0 mmHg, pre-PVR <13.65 Wood units, post-PVR <13.87 Wood units, post-SvO2 ≥63%, and ΔPVR <−0.25 Wood units had significantly better outcomes than other female patients (Figure 7E,F,G). More importantly, an optimal outcome was indicated for those patients who had two prognostic parameters combining at the same time, such as patients with their post-mPAP <53 mmHg and post-SvO2 ≥63%, male patients with their pre-PVR <12.47 Wood units and pre-SvO2 ≥63.6%, or female patients with their pre-mRAP <9.0 mmHg and pre-PVR <13.65 Wood units, or post-PVR <13.87 Wood units and post-SvO2 ≥63%.
Discussion
The present study demonstrated that the acute hemodynamic response to iloprost aerosol provided more information than currently used tests in selecting a minority of patients with PAH who can be treated with CCB. We found that post-mPAP and post-PVR indices were lower than pre-mPAP and pre-PVR indices, while post-CO, post-CI, and post-SaO2 levels were higher than pre-CO, pre-CI, and pre -SaO2 levels after AVT for all patients. More importantly, a pre-CO ≥3.25 L/min, a post-mPAP <53 mmHg, and a post-SvO2 ≥63% had a better prognosis for patients. Pre-PVR <12.47 Wood units, pre-SvO2 ≥64%, and post-mPAP <51 mmHg indicated better outcomes for male patients, whereas ΔmPAP ≥−4 mmHg and ΔPVR ≥−0.25 Wood units indicated better prognosis for female patients. These results may offer an explanation why patients with comparable characteristics have different outcomes during follow-up.
Although IPAH can be managed with targeted drugs (such as endothelin receptor antagonists and prostacyclin inhibitors) which have increased the survival rate of patients with IPAH and improved their outcome, the long-term prognosis of IPAH remains poor (13). Traditionally, IPAH has been considered a disease predominantly affecting women (14,15). Several reasons for the higher female prevalence have been proposed including the role of sex hormones (16,17) and mitochondria (18). Previous studies have suggested that women with IPAH have better survival rates than men (19,20). As the care of patients with IPAH is complex and women with IPAH have a better survival than men, it is important to be able to identify sex-specific prognostic parameters from AVT during RHC.
Our results confirmed the previously hypothesized correlation between a number of baseline hemodynamic parameters, such as higher mRAP, lower CO as well as CI, and worse outcomes (21-23). Other conventionally measured parameters such as mPAP and PVR have been inconsistently related to prognosis (24,25). Our study found that both male and female patients had higher mPAP and PVR levels in non-survivors than in survivors. Furthermore, we found that ΔmPAP and ΔPVR decreased less in female non-survivors than in female survivors after AVT, whereas there was no significant difference in these parameters between male non-survivors and male survivors. Hemodynamic parameters, including the mPAP and PVR, are related not only to the right ventricular afterload but also to the disease burden in the pulmonary vascular bed (24,26,27). Taken together, these results suggest that women have more vasodilatory reserve than men, which may also account for the better prognosis of female patients.
Subgroup analysis showed that female survivors had significantly higher pre-SaO2, post-SaO2, and ΔPVR levels than male survivors, and female non-survivors had higher pre-SaO2, ΔCO, and ΔCO/CO indices than male non-survivors. These results reflect a better reversibility in female patients than male patients, and differs from the sex differences observed in the hemodynamic parameters of AVT in patients with CTEPH as reported in our previous study (8). This might reflect the different pathogenic mechanisms of IPAH and CTEPH where discrete pathophysiological processes could be involved, which may explain this difference in reversibility of the hemodynamic parameters. This also suggests the importance of hemodynamic evaluation in differentiating diseases with similar manifestations.
Many studies have shown the prognostic value of mPAP and CO measures (20,21,25,28,29), and this is in accord with our findings in the present study. In our study, we found that pre-CO ≥3.25 L/min and post-mPAP <53 mmHg were independent prognostic parameters. In addition, we identified other hemodynamic parameters that were correlated with patient prognosis and these differed according to sex. This might be related to the difference in hormone levels between males and females. Further studies are required to confirm this speculation.
SvO2, a parameter related to oxygen delivery and oxygen consumption, was also hypothesized to be a robust indicator of right ventricular function. It provides important prognostic information in many subsets of PH (27,30,31). As expected, pre-SvO2 was identified as an independent predictor of non-survival of male patients with IPAH. However, post-SvO2 was also identified as an independent predictor of non-survival of all patients with IPAH. These results were different from those of our previous study which analyzed the hemodynamic parameters of patients with CTEPH according to sex, and which recommended using SvO2 to predict the prognosis of female patients with CTEPH, and ΔSvO2 to predict the prognosis of male patients with CTEPH (8). This discrepancy in results might be explained by the different sample sizes of the selected populations and the different type of disease. The etiology of CTEPH includes cancer, inflammation, infection, and other specific clinical conditions underlying the failure of thrombus removal. Thrombotic materials impair blood flow, and ultimately lead to the development of CTEPH (32-34). IPAH is the consequence of the progressive increase in PVR due to pulmonary vasoconstriction and structural changes. Pulmonary vasoconstriction is reversible in response to vasodilators, while chronic remodeling of pulmonary vessels is possibly irreversible. Reversibility of pulmonary vasculature is closely related to the severity of the underlying pathology. Therefore, the outcomes for patients with IPAH may depend on the reversibility of the pulmonary vasculature which was directly reflected in the parameters of AVT.
In 2015, Leuchte et al. (6) reported that Δ PVR during AVT was of prognostic relevance for 66 patients with IPAH who presented with a negative acute response. They found that post-CO, post- CI, and post- SvO2 parameters were increased, while post-mPAP and post-PVR parameters were decreased after AVT, but the prognostic value of these parameters was not further explored. In our study, we enrolled a total of 487 patients with IPAH who presented with a negative acute response, the sample size was about 7 times larger than their sample size, which obviously reduced the data bias. We analyzed the above parameters, the changing trends of post-CO, post- CI, and post- SvO2 were consistent with their results. Moreover, we also found that the change of post-SaO2 was statistically significant after inhalation of iloprost aerosol. In addition, we also performed Kaplan-Meier analysis to confirm whether or not these parameters can predict the survival rate. The data indicated that pre-PVR was an independent predictor of prognosis in both male and female patients. Also, post-PVR <13.87 Wood units and ΔPVR <−0.35 Wood units implied a better prognosis. In our study, ΔPVR was associated with prognosis only in female patients, while in the study of Leuchte et al., ΔPVR was a prognostic parameter for all patients. This discrepancy may be due to the different demographic, regional attribution and baseline clinical characteristics of patients.
The cut-off values of the independent predictors, which were determined by using the ROC curve, led to marked differences in survival between subgroups divided by these cut-off values. More subgroups were derived when two independent predictors were combined to further evaluate the power of these predictors in estimating outcomes of patients with IPAH. This method of combining predictors might provide more clues about the outcomes of such patients in clinical practice. More importantly, we found that prognostic predictors differed between male and female patients. The previous studies indicated that better baseline hemodynamic parameters, poor prognosis in men compared with women with PAH (19,35). And female sex is associated with a lower prevalence and a better outcome of adult patients with heart failure (36). In addition, myocardial adaptations to increased afterload differ between sexes, with male subjects possessing a greater tendency to develop left ventricular dilatation and hypertrophy during the course of left ventricular dysfunction (37). Therefore, male and female IPAH patients had not the absolutely consistent disease progression and outcomes, which lead to get different prognostic predictors after regression analyses. Additionally, whether sex hormones contribute to this finding in IPAH also needs further research.
Limitation
There are a number of limitations in the present study. we might have missed responders to other vasodilators such as inhaled nitrous oxide (iNO), rather than the drug we used, However, iloprost aerosol seems to have more pronounced hemodynamic effects on patients with PAH compared to iNO (38). Secondly, we could have missed responders to higher doses of iloprost aerosol during AVT. However, an iloprost aerosol dosage of 5 mg was considered a standard dose for classical vasoresponders by Jing et al. (39) and has been used in various settings before, so it is less likely that we have missed responders at this dose. Thirdly, the present results were obtained from our single center setting and may reflect some selection bias, although it should be noted that our sample size is far larger than other single-center trials to date.
Conclusions
Our study demonstrates for the first time that different hemodynamic parameters of pre-AVT, post-AVT, and ΔAVT have discrete values in predicting the prognosis of patients with IPAH. Sex differences were identified in these parameters, and indicated that both sexes have their own unique hemodynamic parameters that are able to predict outcome. These results suggest that the sex of the patient should be taken into account when estimating prognosis via AVT in IPAH.
Acknowledgments
We extend appreciation to the families who participated in this study and to the team facilitating this research.
Funding: This study was supported by grants from the National Natural Science Foundation of China (81870042, 81700045 and 81900050), National Science and Technology Information System of the People’s Republic of China (2018YFC1313603), Program of Shanghai Science and Technology Committee (201409004100).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-7339
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-7339
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-7339). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study complied with the Declaration of Helsinki (as revised in 2013) and was approved by the Medical Ethics Committee of Shanghai Pulmonary Hospital. Written informed consent was obtained from either the patients or their next of kin.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation 2006;114:1417-31. [Crossref] [PubMed]
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [Crossref] [PubMed]
- Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903-75. [Crossref] [PubMed]
- Chazova IE, Avdeev SN, Tsareva NA, et al. Clinical guidelines for the diagnosis and treatment of pulmonary hypertension. Ter Arkh 2014;86:4-23. [PubMed]
- Leuchte HH, Baezner C, Baumgartner RA, et al. Residual pulmonary vasodilative reserve predicts outcome in idiopathic pulmonary hypertension. Heart 2015;101:972-6. [Crossref] [PubMed]
- Yu YZ, Yuan P, Yang YL, et al. Changed hemodynamics in acute vasoreactivity testing: prognostic predictors in chronic thromboembolic pulmonary hypertension. Am J Transl Res 2020;12:959-73. [PubMed]
- Yang YL, Yu YZ, Yuan P, et al. Sex differences of hemodynamics during acute vasoreactivity testing to predict the outcomes of chronic thromboembolic pulmonary hypertension. Clin Respir J 2020;14:611-21. [Crossref] [PubMed]
- Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC). European Respiratory Society (ERS). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34:1219-63. [Crossref] [PubMed]
- Shigeta A, Tanabe N, Shimizu H, et al. Gender differences in chronic thromboembolic pulmonary hypertension in Japan. Circ J 2008;72:2069-74. [Crossref] [PubMed]
- Yuan P, Ni HJ, Chen TX, et al. Sex-specific cardiopulmonary exercise testing parameters as predictors in patients with idiopathic pulmonary arterial hypertension. Hypertens Res 2017;40:868-75. [Crossref] [PubMed]
- Wang L, Jin YZ, Zhao QH, et al. Hemodynamic and gas exchange effects of inhaled iloprost in patients with COPD and pulmonary hypertension. Int J Chron Obstruct Pulmon Dis 2017;12:3353-60. [Crossref] [PubMed]
- Emanuel R, Chichra A, Patel N, et al. Neurohormonal modulation as therapeutic avenue for right ventricular dysfunction in pulmonary artery hypertension: till the dawn, waiting. Ann Transl Med 2018;6:301. [Crossref] [PubMed]
- Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010;137:376-87. [Crossref] [PubMed]
- Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023-30. [Crossref] [PubMed]
- Foderaro A, Ventetuolo CE. Pulmonary Arterial Hypertension and the Sex Hormone Paradox. Curr Hypertens Rep 2016;18:84. [Crossref] [PubMed]
- Mair KM, Wright AF, Duggan N, et al. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med 2014;190:456-67. [Crossref] [PubMed]
- Marshall JD, Bazan I, Zhang Y, et al. Mitochondrial dysfunction and pulmonary hypertension: cause, effect, or both. Am J Physiol Lung Cell Mol Physiol 2018;314:L782-96. [Crossref] [PubMed]
- Shapiro S, Traiger GL, Turner M, et al. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest 2012;141:363-73. [Crossref] [PubMed]
- Weatherald J, Boucly A, Chemla D, et al. Prognostic Value of Follow-Up Hemodynamic Variables After Initial Management in Pulmonary Arterial Hypertension. Circulation 2018;137:693-704. [Crossref] [PubMed]
- Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156-63. [Crossref] [PubMed]
- Campo A, Mathai SC, Le Pavec J, et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med 2010;182:252-60. [Crossref] [PubMed]
- Saggar R, Sitbon O. Hemodynamics in pulmonary arterial hypertension: current and future perspectives. Am J Cardiol 2012;110:9S-15S. [Crossref] [PubMed]
- McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 2002;106:1477-82. [Crossref] [PubMed]
- Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122:164-72. [Crossref] [PubMed]
- Shapiro S, Traiger GL, Turner M, et al. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest 2012;141:363-73. [Crossref] [PubMed]
- Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002;40:780-8. [Crossref] [PubMed]
- McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol 2013;62:D73-81. [Crossref] [PubMed]
- Vonk Noordegraaf A, Westerhof BE, Westerhof N. The Relationship Between the Right Ventricle and its Load in Pulmonary Hypertension. J Am Coll Cardiol 2017;69:236-43. [Crossref] [PubMed]
- McLaughlin VV, Sitbon O, Badesch DB, et al. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J 2005;25:244-9. [Crossref] [PubMed]
- Sitbon O, McLaughlin VV, Badesch DB, et al. Survival in patients with class III idiopathic pulmonary arterial hypertension treated with first line oral bosentan compared with an historical cohort of patients started on intravenous epoprostenol. Thorax 2005;60:1025-30. [Crossref] [PubMed]
- Lang IM, Dorfmuller P, Vonk Noordegraaf A. The Pathobiology of Chronic Thromboembolic Pulmonary Hypertension. Ann Am Thorac Soc 2016;13 Suppl 3:S215-21. [Crossref] [PubMed]
- Banks DA, Pretorius GV, Kerr KM, et al. Pulmonary endarterectomy: part I. Pathophysiology, clinical manifestations, and diagnostic evaluation of chronic thromboembolic pulmonary hypertension. Semin Cardiothorac Vasc Anesth 2014;18:319-30. [Crossref] [PubMed]
- Wagenvoort CA. Pathology of pulmonary thromboembolism. Chest 1995;107:10S-7S. [Crossref] [PubMed]
- Barbro K, Magnus N, David K, et al. Sex-specific differences and survival in patients with idiopathic pulmonary arterial hypertension 2008-2016. ERJ Open Res 2019;5:00075-2019.
- Melissa RD, Paramjit ST, Naranjan SD. Gender differences in cardiac dysfunction and remodeling due to volume overload. J Card Fail 2010;16:439-49. [Crossref] [PubMed]
- Luchner A, Bröckel U, Muscholl M, et al. Gender-specific differences of cardiac remodeling in subjects with left ventricular dysfunction: a population-based study. Cardiovasc Res 2002;53:720-7. [Crossref] [PubMed]
- Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013;369:809-18. [Crossref] [PubMed]
- Jing ZC, Jiang X, Han ZY, et al. Iloprost for pulmonary vasodilator testing in idiopathic pulmonary arterial hypertension. Eur Respir J 2009;33:1354-60. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)


