The importance of intake: a gut feeling
Under normal circumstances, the stool contains negligible amounts of sodium, indicating that virtually all sodium that is ingested and that is secreted by the gut itself is being reabsorbed. Why is enteric sodium absorption not regulated? Perhaps, because of the osmotic effect of sodium chloride: the gut cannot generate free water and enteric excretion of excess sodium chloride would cause uncontrolled loss of water in the feces as occurs with diarrhea. As a consequence, mammals must excrete excess sodium reabsorbed in the gut via the kidney. Loss of renal function, or disturbed renal perfusion, therefore causes sodium retention, and hence, when non-osmotic stores are full, osmotic fluid retention and finally increased blood pressure and target organ damage.
Limiting enteric sodium absorption is therefore an attractive option when renal sodium excretion is disturbed (because of disturbed renal perfusion in heart failure, loss of functional nephrons in kidney disease, or both as in cardiorenal disease) (1). An effective approach in the gut appears to be inhibition of the electroneutral Na+/H+ exchangers (NHEs), particular NHE3 (2). In a milestone study fluid retention, blood pressure and target organ injury were limited in subtotally nephrectomized (NPX) rats with cardiorenal syndrome when treated with the NHE3 inhibitor tenapanor. This novel drug worked both in a preventive study design (from the onset of renal ablation) and in a rescue study: 14 days after renal ablation when all disease characteristics (fluid retention, hypertension, albuminuria and left ventricular hypertrophy) were present. Importantly, tenapanor, while increasing fecal sodium content in rats, was practically not absorbed and therefore did not disturb NHE3 systemically. Tenapanor also inhibited sodium absorption and increased fecal sodium content in humans. However, the study does not provide information on the magnitude of volume depletion (acute weight change) or activation of the renin-angiotensin-aldosterone system that can be induced by tenapanor (which appears to be less effective in humans than in rats) as compared to volume depletion by the use of diuretics and/or dietary sodium restriction. Nevertheless, prospective randomized, placebo controlled trials are currently under way in patients with chronic kidney disease (CKD) and end-stage kidney disease (ESKD) (reference numbers NCT01764854 and NCT01847092).
The downside is that the osmotic fecal load will by necessity lead to watery feces or frank diarrhea. A solution would be to combine enteric blockade of NHE3 with intake of enough resin to bind the excess water (3,4), but this would make use of the new drug much less attractive. At present it is also not clear if long term use of tenapanor will affect nutrient absorption. Unfortunately, a complete analysis of fecal composition or even data on body weight change in the NPX rats was not provided in the study by Spencer et al. (2), but luminal fluid mass was doubled at the dose used. The argument that feces were not fully analyzed because the watery feces could not be fully separated from urine in the NPX rats is relevant for the dissection of direct and indirect effects of tenapanor on enteric and renal electrolyte excretion, but not relevant for macronutrient loss in the feces. Indeed, lower creatinine clearance in NPX rats treated with tenapanor vs. placebo, in the presence of less renal injury, suggests some loss of muscle mass, in addition to dampening of hyperfiltration due to volume contraction.
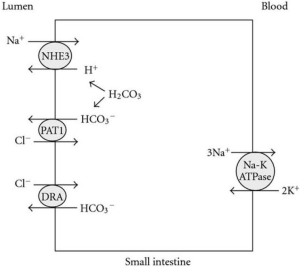
Judging by the changes in urinary sodium and chloride excretion, blocking enteric NHE3 appeared to induce parallel changes in enteric sodium and chloride absorption, suggesting that accumulation of sodium in the gut also increases chloride secretion via a chloride channel. Interestingly, after a nadir at 14 days, both urinary sodium and chloride excretion rates creep towards those of vehicle treated NPX rats, suggesting “escape” of enteric NHE3 blockade, possibly by upregulation of the epithelial sodium channel (ENaC) in the colon. Whether, in long term clinical studies, such “escape” will result in some loss of protective effects of tenapanor remains to be seen. Furthermore, blocking enteric NHE3 results in less H+ secretion, and indeed mild decreases in serum bicarbonate were observed, despite the amelioration of renal damage. In healthy rats there were transient increases in stool potassium, and the area under the curve for urine potassium and urine chloride appeared decreased, but data for these ions is lacking for the NPX rats. Exact mechanisms need to be teased apart but, based on known mechanisms of NHE3-dependent NaCl and H2CO3 exchange in the small intestine (Figure 1) (5), generally these effects were all expected. Clearly, in CKD and ESKD increases in stool potassium are welcome, but fecal loss of bicarbonate will need to be compensated. An interesting development in this context is the introduction of a novel selective cation exchanger that lowers serum potassium (6) while increasing serum bicarbonate (7).
Surprisingly a recent follow-up study by the same group showed that tenapanor induced marked reductions in enteric phosphorus absorption in NPX rats that were on high phosphorus intake in combination with calcitriol supplements (8). This is an accepted model of dystrophic calcification in the setting of CKD (9), and indeed in this model tenapanor also markedly reduced urinary phosphorus excretion as well as serum levels of phosphorus, calcium, creatinine and FGF23. Histology and urine analysis for albumin and KIM-1 revealed marked reductions in renal injury. Moreover, vascular and gastric calcification was practically prevented. About 70% of phosphorus uptake occurs in the small intestine. Of this >50% is due to passive transport secondary to sodium transport and water flux, and <50% is due to active transport mainly via the NaPi-IIb cotransporter (10). Nicotinic acid (niacin) is a drug that inhibits intestinal phosphate absorption by blocking the Na-Pi-2b sodium-dependent phosphate co-transporter (11). The mechanism is unclear, and may simply be the result of less passive phosphorus uptake secondary to the reduced water flux. It remains to be seen whether in other models of CKD-related dystrophic calcification or in humans with CKD similar protection is achieved. Whatever the mechanism, it appears to be entirely different from phosphate binders (8) or nicotinic acid. Furthermore, effect on renal function should not be judged merely by plasma creatinine or urea. Because of possible effects of NHE3 blockers on protein balance (see above), classic measurement of renal function with an exogenous compound, for instance inulin, are required. Nevertheless, these results are all very exciting, particularly in the light of the poor performance of current guidelines for oral phosphate binders in patients with CKD or ESKD (12). However, NHE3 blockers will not have the beneficial effects of reduced intestinal uptake of colon-derived uremic toxins (13), as is the case with conventional phosphate binders that also bind uremic toxins (3).
We have yet to discover the clinical relevance in volume terms and vascular calcifications in patients in relation to the tolerated dose. However, even if the effect of tenapanor is limited in humans, combination therapy with diuretics may be an interesting option in selected patients. All in all, the introduction of tenapanor in treatment of patients with CKD and ESKD, heart failure and cardiorenal syndrome may herald a new era in their care. But, even if expectations are somewhat too high, findings in ongoing and projected studies that manipulate enteric sodium absorption will teach us a lot about the interaction between the gut and the kidney, and effects on non-osmotic sodium stores.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bongartz LG, Braam B, Gaillard CA, et al. Target organ cross talk in cardiorenal syndrome: animal models. Am J Physiol Renal Physiol 2012;303:F1253-63. [PubMed]
- Spencer AG, Labonte ED, Rosenbaum DP, et al. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med 2014;6:227ra36.
- Charmot D. Non-systemic drugs: a critical review. Curr Pharm Des 2012;18:1434-45. [PubMed]
- Simon J, Strickland AD. Inventors, Dow Global Technologies Inc., Assignee: use of water absorbent polymers. United States Patent S6908609B2.2005.
- Soleimani M, Alborzi P. The role of salt in the pathogenesis of fructose-induced hypertension. Int J Nephrol 2011;2011:392708.
- Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015;372:222-31. [PubMed]
- Ash SR, Singh B, Lavin PT, et al. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int 2015. [Epub ahead of print]. [PubMed]
- Labonté ED, Carreras CW, Leadbetter MR, et al. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 2014. [Epub ahead of print]. [PubMed]
- Haffner D, Hocher B, Müller D, et al. Systemic cardiovascular disease in uremic rats induced by 1,25(OH)2D3. J Hypertens 2005;23:1067-75. [PubMed]
- Sabbagh Y, Giral H, Caldas Y, et al. Intestinal phosphate transport. Adv Chronic Kidney Dis 2011;18:85-90. [PubMed]
- Berns JS. Niacin and related compounds for treating hyperphosphatemia in dialysis patients. Semin Dial 2008;21:203-5. [PubMed]
- Hutchison AJ, Smith CP, Brenchley PE. Pharmacology, efficacy and safety of oral phosphate binders. Nat Rev Nephrol 2011;7:578-89. [PubMed]
- Vanholder R, Schepers E, Pletinck A, et al. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol 2014;25:1897-907. [PubMed]


