Prognostic value of neutrophil to lymphocyte ratio for gastric cancer
Introduction
Gastric cancer (GC) is one of the most prevalent malignant diseases worldwide, accounting for about 8% of all cancers and 10% of cancer-related deaths (1). Although the survival benefit of palliative chemotherapy and surgical treatment in GC has been well recognized, the outcome remains dismal. To improve the outcome of GC patients, prognostic assessment is a critical, for it can affect decision-making for GC treatment (2). Generally, prognostic assessment is based on various prognostic factors, among which the most well established include TNM stage (3,4), pathohistological classification (5), resection margin (6), serosal invasion (6), inflammation factors (7), and tumor markers (8-10). Although there are various prognostic factors available, some of them are invasive and/or cannot be acquired before treatment, and therefore their value in clinical practice is limited to some extent, especially during the initial phase of GC treatment.
Inflammation is known to be involved in the occurrence and development of GC (11-13), and inflammatory markers such as C-reactive protein (CRP) (14,15) and the erythrocyte sedimentation rate (ESR) (16) have been regarded as the useful diagnostic or prognostic markers for GC. As most inflammatory markers are non-invasive and can be acquired before treatment, their prognostic value has aroused much interest.
The neutrophil to lymphocyte ratio (NLR) is a well-recognized inflammatory index and has been reported as a useful prognostic index (PI) in GC in many studies. However, the results remain inconsistent. The objective of this systematic review is to ascertain the prognostic value of NLR for GC.
Materials and methods
Literature searching strategy
Two authors independently searched the electronic databases including PubMed and Embase to identify potential studies. The last search was performed on 8 June, 2014. The search terms for PubMed were: (“neutrophil/lymphocyte” or “neutrophil to lymphocyte” or “neutrophil lymphocyte” OR “neutrophil-lymphocyte”) AND (“stomach cancer” OR “gastric cancer” OR “Stomach Neoplasms [MESH]” OR “stomach malignant”). Similar strategies were used for EMBASE. Manual searches were also performed by reviewing the references listed at the end of eligible studies.
Study selection
The inclusion criteria for the present systematic review were as follows: (I) studies that evaluated the prognostic value of NLR in GC patients; (II) studies with a follow-up duration longer than 6 months; (III) studies reporting at least one of the following endpoints: overall survival (OS), progression-free survival (PFS), disease-free survival (DFS) and disease special survival (DSS). The following were excluded: (I) animal studies; (II) conference abstracts; (III) duplicated publications; and (IV) manuscripts in languages other than English. Screening of eligible studies was conducted in two steps: first, two authors independently reviewed the abstracts and titles of the retrieved studies to identify potentially eligible studies, and then reviewed their full texts. Any disagreement in study selection was resolved by discussions.
Data extraction
Two authors independently performed data extraction and quality assessment. The following data were extracted: name of the first author, publication year, participant characteristics, sample size, follow-up duration, endpoint and corresponding hazard ratio (HR) and 95% confidence interval (CI), and confounding factors adjusted. If more than one HR was provided in an individual study, the most fully adjusted HR was extracted. The corresponding authors of the eligible studies were not contacted for further information.
The Cochrane recommended Newcastle-Ottawa Scale (NOS) (17) for cohort study was used for quality assessment, with minor modifications. This tool consisted of three domains: selection domain (maximum: four score), comparability (maximum: two score), and outcome (maximum: three score).
Any disagreement in data extraction and quality assessment was resolved by discussions among all authors.
Results
Summary of eligible studies
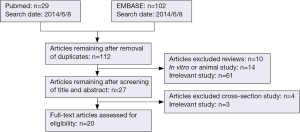
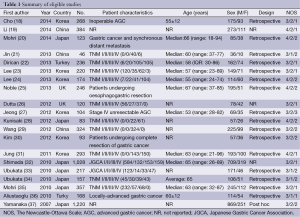
Figure 1 indicates the flowchart describing our literature search. A total of 20 studies (18-37) were included and the summaries of eligible studies are shown in Table 1. Since none of the eligible studies declared that they reported the results according to the reporting recommendations for tumor marker prognostic studies (REMARK) (38), some of the design details or results were not reported and thus labeled as “not reported (NR)”. Seven studies were from Japan (20,28,32-35,37), six from Korea (18,23,24,27,30,31), three from China (19,21,29), two from Turkey (22,36), and two from UK (25,26). The sample size ranged from 46 to 1,220. Eight studies were conducted in patients with advanced GC patients (stage III or IV) (18,20,21,27-29,31,36), eight in all stages of GC (22-24,26,32-35), and two in GC patients undergoing surgical resection (25,30). Two studies did not report the characteristics of GC patients (19,37). Generally, the male/female ratio in GC cohorts arranged approximately from 1.5 to 4, and the mean or median age was older than 50 years. Sixteen studies (18,20-25,27-31,33-36) were retrospective, one study (37) was post hoc analysis, and three studies (19,26,32) did not report how they collected the data.
Full table
Quality assessment of eligible studies
Generally, the selection domains of all eligible studies were good, including 14 studies (18,21-23,26,27,29,30,32-37) that were labeled as three because the authors did not report how they enrolled the patients (consecutively, randomly or neither). Comparability domains in four studies (21,33,34,36) were labeled as one because the confounding factors were not fully taken into consideration. Outcome domains in most of the eligible studies were label as one or two because: (I) the authors did not report how to get the information of endpoints (18,19,21-23,27,30-36); and/or (II) did not report the follow-up rate (18,30,32,37), or the follow-up rate was lower than 80% (19,20,22,23,25,28,29,31,32,35,36). The score of each domain is listed in Table 1.
Major findings of eligible studies
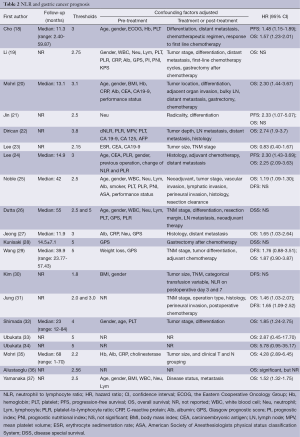
As shown in Table 2, 10 (19,21-23,30,31,33,34,36,37) of the 20 eligible studies did not report the follow-up duration, and the median follow-up duration of the remaining studies arranged from 11 to 68 months. The threshold of NLR used to categorize GC patients ranged from 1.8 to 5.
Full table
OS is the most widely used endpoint of cohort studies. Seventeen studies (18-25,27,29,31-37) analyzed the association between NLR and OS, of which 11 studies (18,20,22,24,25,27,31,32,35-37) found that increased NLR was associated with a higher all-cause mortality, and the remaining six studies (19,21,23,29,33,34) failed to observe the association between NLR and OS. However, univariate analysis of 16 studies (18,19,21-25,27,29,31-37) showed that increased NLR was associated with worse OS. PFS was set as the endpoint in three studies (18,21,24), and the association between increased NLR and worse PFS was observed in two (18,21). DFS was set as the endpoint in four studies (25,29-31), and two (25,31) of them found that increased NLR was associated with worse DFS. DSS was set as the endpoint in two studies (26,28), and neither concluded that NLR could affect DSS.
The confounding factors, including pre-, intra- and post-treatment ones, were various in the eligible studies. The most common pretreatment factors adjusted in eligible studies were age, gender, platelet count, CA19-9, body mass index (BMI) or weight loss, CRP, albumin, ESR, carcinoembryonic antigen (CEA), PI, prognostic nutritional index (PNI) and the Eastern Cooperative Oncology Group (ECOG). The most common intra-treatment or post-treatment factors adjusted were tumor differentiation, adjuvant chemotherapy, tumor stage or location, operation type, histology or perineural invasion.
Meta-analysis was not performed to further investigate the prognostic value of NLR due to the following reasons: (I) the subjects were heterogeneous in all eligible studies, especially in tumor stage and treatment strategies; (II) the threshold to categorize GC patients was heterogeneous; (III) the confounding factors adjusted in all eligible studies were heterogeneous; and (IV) HR was not reported in some of the eligible studies, especially the studies with negative findings.
Discussion
The present systematic review investigated the prognostic value of NLR for GC. We found that the 20 studies available were heterogeneous in subjects, statistical analysis and data presentation. Most of the eligible studies reported that NLR was a useful index to estimate the OS, PFS and DFS, but not DSS. In addition, 16 of the 17 studies that investigated the effect of NLR on OS reported that increased NLR was associated with worse OS in univariate analysis, suggesting that NLR is a useful index to estimate the prognosis of GC. In addition to the major findings of the studies available, some methodological problems should not be ignored and need to be carefully addressed in future research.
The first methodological problem is the bias of participant selection. It should be noted that all the eligible studies were retrospective and only six (19,20,24,25,28,31) out of the 20 studies reported that they included the entire potential eligible subjects admitted in the hospitals. As subject enrollment in a retrospective study largely depends on the completeness of medical records, some potentially eligible patients without complete medical records may be excluded from the study, which may introduce participant selection bias. To avoid cohort selection bias, consecutive enrollment for subjects is essential. Unfortunately, only five studies reported that they enrolled their subjects consecutively.
The second methodological problem is the process of data analysis. All but one (26) categorized the subjects into two groups with variable thresholds. Although this is a common approach used by most retrospective studies, it may cause a great information loss (39). The threshold used to transform continuous data into binary data has a great effect on the result of the Cox model (39,40). It would be better to investigate the prognostic value of NLR in three or four groups rather than just in two groups (39). In addition to the threshold to categorize continuous variables, confounding factors constitute another methodological problem in data analysis. Many of these confounding factors were adjusted in the Cox model, and categorized into three types: pre-, intra- or post-treatment as shown in Table 2. Some of the eligible studies found that NLR had no independent effect on the prognosis of GC, although univariate analysis showed that increased NLR was associated with the worse prognosis of GC. Statistically, all the potential confounding factors should be adjusted for analysis. But clinically, noninvasive prognostic factors should be preferred for easy acquisition, especially those obtained before treatment because they can affect the establishment of initial treatment strategies. In other words, it is valuable to see whether the prognostic value of intra- or post-treatment factors is independent of pretreatment factors, but not the opposite. Among the well-recognized pre-treatment prognostic factors, NLR may be preferable because it is non-invasive and inexpensive
High neutrophil counts have long been reported to negatively affect the prognosis of GC (41), probably because they could promote the proliferation, invasion and angiogenesis of cancer by producing various factors, and effectively suppress anti-tumor response initiated by the immune system (42). On the other hand, low lymphocyte counts are believed to be associated with worse prognosis in various types of cancer because they weaken the lymphocyte-mediated anti-tumor cellular immune response, and also reflect the status of malnutrition in GC patients. NLR, defined as the absolute number of neutrophils divided by the absolute number of lymphocytes, has incorporated the prognostic value of neutrophils and lymphocytes, and therefore may better reflect the prognosis of GC.
In summary, the present systematic review suggests that NLR is a useful, inexpensive and noninvasive pre-treatment prognostic factor for GC. Since most studies in the present systematic review are retrospective, we call for prospective studies with larger sample sizes to rigorously assess the prognostic value of NLR for GC in future.
Acknowledgements
We thank Dr. Zhi-Rui Zhou (Department of Radiation Oncology, Tumor Hospital of Jilin Province) for helpful discussion. This study program was supported by grants from the National Natural Science Foundation of China [81302541].
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Thrumurthy SG, Chaudry MA, Hochhauser D, et al. The diagnosis and management of gastric cancer. BMJ 2013;347:f6367. [PubMed]
- Graziosi L, Marino E, Cavazzoni E, et al. Prognostic value of the seventh AJCC/UICC TNM classification of non-cardia gastric cancer. World J Surg Oncol 2013;11:103. [PubMed]
- Marrelli D, Morgagni P, de Manzoni G, et al. Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized Western centers. Ann Surg 2012;255:486-91. [PubMed]
- Berlth F, Bollschweiler E, Drebber U, et al. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol 2014;20:5679-84. [PubMed]
- Shiraishi N, Sato K, Yasuda K, et al. Multivariate prognostic study on large gastric cancer. J Surg Oncol 2007;96:14-8. [PubMed]
- Yeh YC, Sheu BS, Cheng HC, et al. Elevated serum matrix metalloproteinase-3 and -7 in H. pylori-related gastric cancer can be biomarkers correlating with a poor survival. Dig Dis Sci 2010;55:1649-57. [PubMed]
- Choi AR, Park JC, Kim JH, et al. High level of preoperative carbohydrate antigen 19-9 is a poor survival predictor in gastric cancer. World J Gastroenterol 2013;19:5302-8. [PubMed]
- Xiao J, He X, Wang Z, et al. Serum carbohydrate antigen 19-9 and prognosis of patients with gastric cancer. Tumour Biol 2014;35:1331-4. [PubMed]
- Huang ZB, Zhou X, Xu J, et al. Prognostic value of preoperative serum tumor markers in gastric cancer. World J Clin Oncol 2014;5:170-6. [PubMed]
- Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology 2012;143:550-63. [PubMed]
- Oguma K, Oshima H, Oshima M. Inflammation, tumor necrosis factor and Wnt promotion in gastric cancer development. Future Oncol 2010;6:515-26. [PubMed]
- Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest 2007;117:60-9. [PubMed]
- Zhang P, Zou M, Wen X, et al. Development of serum parameters panels for the early detection of pancreatic cancer. Int J Cancer 2014;134:2646-55. [PubMed]
- Yu Q, Yu XF, Zhang SD, et al. Prognostic role of C-reactive protein in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev 2013;14:5735-40. [PubMed]
- Janssen CW Jr, Maartmann-Moe H, Lie RT. Preoperative prediction of extent and prognosis of gastric carcinoma by four serum proteins and erythrocyte sedimentation rate. Eur J Surg Oncol 1987;13:285-95. [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Cho IR, Park JC, Park CH, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer 2014;17:703-10. [PubMed]
- Li QQ, Lu ZH, Yang L, et al. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev 2014;15:945-50. [PubMed]
- Mohri Y, Tanaka K, Ohi M, et al. Identification of prognostic factors and surgical indications for metastatic gastric cancer. BMC Cancer 2014;14:409. [PubMed]
- Jin H, Zhang G, Liu X, et al. Blood neutrophil-lymphocyte ratio predicts survival for stages III-IV gastric cancer treated with neoadjuvant chemotherapy. World J Surg Oncol 2013;11:112. [PubMed]
- Dirican A, Ekinci N, Avci A, et al. The effects of hematological parameters and tumor-infiltrating lymphocytes on prognosis in patients with gastric cancer. Cancer Biomark 2013;13:11-20. [PubMed]
- Lee DY, Hong SW, Chang YG, et al. Clinical significance of preoperative inflammatory parameters in gastric cancer patients. J Gastric Cancer 2013;13:111-6. [PubMed]
- Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer 2013;13:350. [PubMed]
- Noble F, Hopkins J, Curtis N, et al. The role of systemic inflammatory and nutritional blood-borne markers in predicting response to neoadjuvant chemotherapy and survival in oesophagogastric cancer. Med Oncol 2013;30:596. [PubMed]
- Dutta S, Crumley AB, Fullarton GM, et al. Comparison of the prognostic value of tumour and patient related factors in patients undergoing potentially curative resection of gastric cancer. Am J Surg 2012;204:294-9. [PubMed]
- Jeong JH, Lim SM, Yun JY, et al. Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology 2012;83:292-9. [PubMed]
- Kunisaki C, Takahashi M, Ono HA, et al. Inflammation-based prognostic score predicts survival in patients with advanced gastric cancer receiving biweekly docetaxel and s-1 combination chemotherapy. Oncology 2012;83:183-91. [PubMed]
- Wang DS, Ren C, Qiu MZ, et al. Comparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancer. Tumour Biol 2012;33:749-56. [PubMed]
- Kim YH, Choi WJ. The effectiveness of postoperative neutrophils to lymphocytes ratio in predicting long-term recurrence after stomach cancer surgery. J Korean Surg Soc 2012;83:352-9. [PubMed]
- Jung MR, Park YK, Jeong O, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol 2011;104:504-10. [PubMed]
- Shimada H, Takiguchi N, Kainuma O, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer 2010;13:170-6. [PubMed]
- Ubukata H, Konishi S, Nakachi T, et al. Characteristics of the serum pepsinogen (Pg) test, and the relationship between Pg test results and gastric cancer outcomes. Scand J Surg 2010;99:201-7. [PubMed]
- Ubukata H, Motohashi G, Tabuchi T, et al. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol 2010;102:742-7. [PubMed]
- Mohri Y, Tanaka K, Ohi M, et al. Prognostic significance of host- and tumor-related factors in patients with gastric cancer. World J Surg 2010;34:285-90. [PubMed]
- Aliustaoglu M, Bilici A, Ustaalioglu BB, et al. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol 2010;27:1060-5. [PubMed]
- Yamanaka T, Matsumoto S, Teramukai S, et al. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology 2007;73:215-20. [PubMed]
- McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 2005;93:387-91. [PubMed]
- Altman DG, Lausen B, Sauerbrei W, et al. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994;86:829-35. [PubMed]
- Altman DG. Categorising continuous variables. Br J Cancer 1991;64:975. [PubMed]
- Bruckner HW, Lavin PT, Plaxe SC, et al. Absolute granulocyte, lymphocyte, and moncyte counts. Useful determinants of prognosis for patients with metastatic cancer of the stomach. JAMA 1982;247:1004-6. [PubMed]
- Galdiero MR, Bonavita E, Barajon I, et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013;218:1402-10. [PubMed]