
The emergence of various genetic alterations mediated the Osimertinib resistance of a patient harboring heterozygous germline EGFR T790M: a case report
Introduction
Despite harboring activating mutations in epidermal growth factor receptor (EGFR), 20–30% of patients with EGFR-mutant non-small cell lung cancer (NSCLC) lack an objective response or only respond to EGFR tyrosine kinase inhibitor (TKI) therapy for less than 3 months due to the existence of genetic alterations that mediate primary resistance to EGFR-TKIs (1,2). EGFR T790M is commonly acquired during EGFR-TKI therapy and accounts for the majority of secondary resistance to first- and second-generation EGFR-TKI; however, 2% of patients harbor either somatic or germline T790M before any exposure to EGFR-TKIs, resulting in primary resistance (1,3,4). Germline EGFR T790M has been reported in non-smokers and is associated with inherited lung cancer susceptibility (3-6). The EGFR activating mutation rate is between 40–50% among Chinese NSCLC patients (7); however, germline EGFR T790M is rare (6,8). EGFR T790M-mediated resistance can effectively be reversed by the third-generation EGFR-TKI Osimertinib (9,10). Similar to clinical findings about the benefits of Osimertinib therapy for patients with acquired T790M from first- or second-generation EGFR-TKI therapy (9,10), first-line Osimertinib therapy has been shown to benefit NSCLC patients who harbor either somatic T790M at baseline or germline T790M with concomitant EGFR sensitizing mutation (8,11). In this paper, we discuss a Chinese patient with advanced lung adenocarcinoma harboring germline EGFR T790M who developed Osimertinib resistance despite a durable partial response. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-7626).
Case presentation
In October 2018, a 57-year-old non-smoking female patient presented with an intermittent dry cough and chest tightness at our institution. Enhanced computed tomography (CT) scans of the patient’s chest revealed soft-tissue nodules in the basal segment of the right lobe of the lung, the presence of bilateral ground-glass nodules, and the slight enlargement of the right hilar and mediastinal lymph nodes (Figure 1A). The patient was diagnosed with Stage IV (T4N2M1a) lung adenocarcinoma. Following the guidelines for molecular testing for all lung cancer patients, tissue biopsy samples were sent for molecular testing. An allele-specific polymerase chain reaction assay was used to confirm the EGFR L858R mutation, after which the patient was administered with icotinib at the standard daily dose of 125 mg achieving stable disease (Figure 1B).

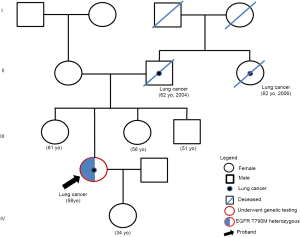
A review of the patient’s CT scans at 3 months revealed the enlargement of primary lung lesions, evaluated as progressive disease by the investigators (Figure 1C). Plasma samples collected at icotinib progression were sent for targeted sequencing with a panel consisting of 168 genes (Burning Rock Biotech, Guangzhou, China). The analysis of the plasma sample only detected EGFR T790M. The targeted sequencing of the archived tissue biopsy sample from baseline detected EGFR L858R, EGFR T790M, and catenin beta 1 (CTNNB1) S37F. Interestingly, the allelic fraction (AF) of EGFR T790M detected from both the tissue and plasma samples consistently approximated 50% (Figure 1), which raised the suspicion that the T790M was a germline mutation. EGFR testing of the lymphocyte genomic DNA confirmed the germline heterozygous status of the T790M mutation. Further investigations of the patient’s family history revealed that the patient’s father and paternal aunt had died of lung cancer in 2004 and 2006, respectively (Figure 2). Her living family members were currently all cancer-free. Osimertinib was then administered at 80 mg once daily starting from April 1, 2019. Within two months of Osimertinib therapy, a 33.6% shrinkage of the primary lesions was observed, and was deemed by the investigators to be a partial response based on the Response Evaluation Criteria in Solid Tumors Version 1.1 (Figure 1D,E). No adverse events were observed. On June 23, 2020, after approximately 15.2 months of Osimertinib therapy, the primary lesions remained stable; however, the accumulation of pleural fluid was observed on the left lung lobe, indicating disease progression (Figure 1F). The targeted sequencing of the pleural effusion and plasma samples collected at Osimertinib progression identified the EGFR T790M and the emergence of various mutations, including EGFR G719A, tumor protein p53 (TP53) Q136X, and the co-amplification of Cyclin D1 (CCND1), fibroblast growth factor (FGF) 19, FGF3, and FGF4 (Figure 1). Pleural effusion cytology showed adenocarcinoma features.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient. The patient has given her consent for the publication of her case.
Discussion
Primary resistance is defined as a lack of tumor regression after 3 months of uninterrupted EGFR-TKI therapy (1). Numerous studies have associated various genetic alterations in the Kirsten rat sarcoma viral oncogene homolog (KRAS) (12), mesenchymal-to-epithelial transition (MET) (1), anaplastic lymphoma kinase (ALK) (1), human epidermal growth factor receptor 2 (HER2) (6) and in the kinase domain of EGFR, including T790M (1,6), as playing an important role in mediating primary resistance to EGFR-TKI. These genetic alterations impair the response of and confer resistance to inhibition via EGFR-dependent and EGFR-independent signaling pathways similar to acquired mechanisms of EGFR-TKI resistance (13). Compared to acquired EGFR-TKI resistance, the molecular mechanisms mediating primary resistance are less well understood.
In our patient, the disease control for 3 months of icotinib therapy was mediated by the subpopulation of EGFR L858R-mutant clones. However, due to the existence of germline EGFR T790M, icotinib had limited activity. However, subsequent Osimertinib therapy was able to overcome the primary resistance to icotinib by targeting the tumor cells harboring the T790M mutation, resulting in remarkable tumor regression. Interestingly, both the plasma and tissue biopsy samples from our patient consistently harbored the T790M mutation at an AF of approximately 50%, indicating a heterozygous germline mutation. In support of these findings, another study also demonstrated the accuracy of plasma genotyping in identifying the germline status of T790M as a heterozygous or homozygous mutation if the AF approximates 50% or 100%, respectively, which is far greater than the AF of common EGFR driver mutations (14). The heterozygosity of the T790M mutation and the patient’s family history of lung cancer strongly suggested inherited lung cancer in an autosomal dominant inheritance pattern. However, the mutation status of the deceased father and paternal aunt were unavailable.
Lung cancer typically develops sporadically through the acquisition of somatic mutations; conversely, inherited lung cancer is rare (6). Due to the possibility of familial lung cancer predisposition associated with germline T790M, and as half of the patients who harbor T790M at baseline are carriers of the germline T790M mutation, the National Comprehensive Cancer Network guidelines recommend additional testing and genetic counseling for those in whom T790M has been detected before exposure to EGFR-TKI (15). The low incidence of germline T790M in East Asians may be due to the widespread use of traditional methods of EGFR testing. As targeted sequencing has become integrated as a routine diagnostic procedure in clinical oncology in recent years, it is also likely that baseline detection of T790M will increase. Targeted sequencing facilitates individualized therapy by revealing not only the mutations that impart sensitivity to currently available targeted therapeutics but also other concurrent mutations that could mediate resistance.
EGFR-dependent and -independent mechanisms of Osimertinib resistance have been well elucidated (16); however, the mechanisms of Osimertinib resistance in certain subsets of patients remain unknown. In our patient, three main genetic alterations were acquired during Osimertinib therapy, EGFR G719A, TP53 Q136X, and the amplification of chromosomal region 11q13 that contains CCND1, FGF19, FGF3, and FGF4. At least one of these alterations was the oncogenic driver of disease progression as observed by the development of pleural effusion in the other lobe. In a previous study, durable partial response with front-line Osimertinib therapy was reported in a patient with advanced lung adenocarcinoma who harbored EGFR G719A and germline EGFR T790M (11); thus, EGFR G719A was ruled out as the driver of Osimertinib resistance in our patient. Despite the lack of evidence implicating TP53 Q136X as an EGFR-independent resistance mechanism to Osimertinib or any other EGFR-TKI, this nonsense mutation in the exon 4 of TP53 has been reported as one of the TP53 mutations that confer poor prognoses for patients with EGFR-mutant advanced NSCLC (17). It has been suggested that the detection of concomitant TP53 mutations before the initiation of EGFR-TKI therapy can reduce the efficacy of EGFR-TKIs, leading to a significantly shorter progression-free survival (18). This suggests that TP53 Q136X is one of the contributors in driving disease progression, but not the major driver.
The amplification of chromosomal region 11q13, which contains numerous genes, including CCND1, FGF19, FGF3, and FGF4, has been reported in 5.6% of cancer patients (22/391), a majority of whom had breast cancer (68%, 15/22) (19,20). CCND1 amplification in breast tumors has been associated with increased tumor aggressiveness and implicated in poor prognosis and treatment failure (21). The co-amplification of FGF3, FGF4, FGF19, and CCND1 has been associated with a significantly higher median number of alterations (19), has been found in cancer patients who experienced hyperprogression with immunotherapy, and may accelerate tumor growth (22). FGF and FGF receptor (FGFR) signaling regulates essential biological processes, including wound healing and angiogenesis (19,20). Aberrant FGFR signaling brought about by genetic alterations in FGF/FGFR has been implicated in the development and/or progression of cancer, and has thus been considered as therapeutic targets in various solid tumors (19,20). The co-amplification of FGF3/FGF19/EMSY at chromosome 11q13.3–13.5 locus was detected in a patient with EGFR-mutant lung adenocarcinoma at Osimertinib progression (16). Thus, the evidence suggests that the amplification of chromosome 11q13 plays a role in cancer progression. The exact Osimertinib resistance mechanism could not be pinpointed in our patient; however, the increased genetic heterogeneity could synergistically upregulate EGFR-independent pathways that drive Osimertinib resistance and promote disease progression. We speculate that the selective pressure brought upon by Osimertinib therapy promoted the growth of other clones harboring the various genetic alterations that we identified from the samples obtained at Osimertinib progression.
To the best of our knowledge, this is the first report of the emergence of Osimertinib resistance in a patient harboring germline heterozygous EGFR T790M. This study also highlighted the importance of conducting targeted next-generation sequencing-based molecular testing during both diagnostic and disease progression assessments to gain an understanding of the genetic landscape for therapeutic decisions and treatment monitoring.
Acknowledgments
The authors would like to thank the patient and her family for their participation in this study. We would also like to thank the investigators, study coordinators, operation staff, and the whole project team who worked on this case. We are also grateful to Qiaolin Kang and Yan Zheng of Burning Rock Biotech for their assistance in data collection.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-7626
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-7626). AL is an employee of Burning Rock Biotech. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient. The patient has given her consent for the publication of her case.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee JK, Shin JY, Kim S, et al. Primary resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with non-small-cell lung cancer harboring TKI-sensitive EGFR mutations: an exploratory study. Ann Oncol 2013;24:2080-7. [Crossref] [PubMed]
- Wang J, Wang B, Chu H, et al. Intrinsic resistance to EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer with activating EGFR mutations. Onco Targets Ther 2016;9:3711-26. [Crossref] [PubMed]
- Ma L, Chen R, Wang F, et al. EGFR L718Q mutation occurs without T790M mutation in a lung adenocarcinoma patient with acquired resistance to osimertinib. Ann Transl Med 2019;7:207. [Crossref] [PubMed]
- Bell DW, Gore I, Okimoto RA, Godin-Heymann N, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet 2005;37:1315-6. [Crossref] [PubMed]
- Gazdar A, Robinson L, Oliver D, et al. Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. J Thorac Oncol 2014;9:456-63. [Crossref] [PubMed]
- Yamamoto H, Yatabe Y, Toyooka S. Inherited lung cancer syndromes targeting never smokers. Transl Lung Cancer Res 2018;7:498-504. [Crossref] [PubMed]
- Li M, Zhou CZ, Yang JJ, et al. The in cis compound EGFR mutations in Chinese advanced non-small cell lung cancer patients. Cancer Biol Ther 2019;20:1097-104. [PubMed]
- Huo R, Li J, Li X, et al. Significant Benefits of Osimertinib Against Adenosquamous Carcinoma Harboring Germline T790M Mutation. Oncologist 2020;25:826-32. [Crossref] [PubMed]
- Zhou W, Ercan D, Chen L, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 2009;462:1070-4. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Ancevski Hunter K, Friedland DM, Villaruz LC, et al. First-Line Osimertinib in Patients with Treatment-Naive Somatic or Germline EGFR T790M-Mutant Metastatic NSCLC. J Thorac Oncol 2018;13:e3-e5. [Crossref] [PubMed]
- Takeda M, Okamoto I, Fujita Y, et al. De novo resistance to epidermal growth factor receptor-tyrosine kinase inhibitors in EGFR mutation-positive patients with non-small cell lung cancer. J Thorac Oncol 2010;5:399-400. [Crossref] [PubMed]
- Del Re M, Crucitta S, et al. Understanding the Mechanisms of Resistance in EGFR-Positive NSCLC: From Tissue to Liquid Biopsy to Guide Treatment Strategy. Int J Mol Sci 2019;20:3951. [Crossref] [PubMed]
- Hu Y, Alden RS, Odegaard JI, et al. Discrimination of Germline EGFR T790M Mutations in Plasma Cell-Free DNA Allows Study of Prevalence Across 31,414 Cancer Patients. Clin Cancer Res 2017;23:7351-9. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology - Non-Small Cell Lung Cancer Version 1.2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Le X, Puri S, Negrao MV, et al. Landscape of EGFR-Dependent and -Independent Resistance Mechanisms to Osimertinib and Continuation Therapy Beyond Progression in EGFR-Mutant NSCLC. Clin Cancer Res 2018;24:6195-203. [Crossref] [PubMed]
- Aggarwal C, Davis CW, Mick R, et al. Influence of TP53 mutation on survival in patients with advanced EGFR-mutant non-small-cell lung cancer. JCO Precis Oncol 2018;2018. [Crossref] [PubMed]
- Iwama E, Sakai K, Azuma K, et al. Exploration of resistance mechanisms for epidermal growth factor receptor-tyrosine kinase inhibitors based on plasma analysis by digital polymerase chain reaction and next-generation sequencing. Cancer Sci 2018;109:3921-33. [Crossref] [PubMed]
- Parish A, Schwaederle M, Daniels G, et al. Fibroblast growth factor family aberrations in cancers: clinical and molecular characteristics. Cell Cycle 2015;14:2121-8. [Crossref] [PubMed]
- Katoh M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat Rev Clin Oncol 2019;16:105-22. [Crossref] [PubMed]
- Holm K, Staaf J, Jonsson G, et al. Characterisation of amplification patterns and target genes at chromosome 11q13 in CCND1-amplified sporadic and familial breast tumours. Breast Cancer Res Treat 2012;133:583-94. [Crossref] [PubMed]
- Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res 2017;23:4242-50. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


