
Clinicopathological characteristics and prognostic factors of pulmonary sarcomatoid carcinoma: a large population analysis
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a collection of five distinct subtypes of lung cancer: pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and pulmonary blastoma (1). PSC is extremely rare, accounting for less than 1% of all lung cancers (2). Advanced stage is a high risk factor for PSC patients. Similar to lung squamous cell cancer and adenocarcinoma patients, PSC patients with advanced age had worse survival (3). TP53 mutations are common in contrast to KRAS and EGFR. Whereas, EGFR mutation is rare in carcinosarcoma, however, patients with EGFR mutation always have better survival outcomes with the use of anti-EGFR treatment (4,5).
PSC remains an understudied sub-type of NSCLC because of its rarity. Few previous studies have compared the clinicopathological characteristics and survival outcomes between the elderly and non-elderly patients. Most researches on PSC are single-institution retrospective studies, and clinical understanding of its biological characteristics is limited. We chose to study PSC using the Surveillance, Epidemiology and End Results (SEER) Program, which is supported by the National Cancer Institute (NCI) and contains the research data of 18 different population-based cancer registries, covering 30% of the United States population (6).
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-6213).
Methods
Data source
Patient data from 2004 to 2016 were extracted from the SEER 18 Database using SEER*Stat software (version 8.3.6). Only patients with a single primary tumor (sequence number =0 or 1) were included, as survival in patients with multiple primary tumors could not be ascribed to a single anatomical cancer site. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study population and inclusion criteria
Patients meet the inclusion criteria as following were included in this study: the International Classification of Diseases for Oncology, 3rd edition codes (ICD-O-3 Codes) were including pleomorphic carcinoma (8022/3), giant cell carcinoma (8031/3), spindle cell carcinoma (8032/3), pulmonary blastoma (8972/3), and carcinosarcoma (8980/3). Information regarding race, age, gender, primary site, histology subtypes, stage, year of diagnosis, treatment and survival data were collected.
The exclusion criteria were as follows: (I) patients with missing or incomplete survival data; (II) patients with histology and no pathological confirmation; and (III) patients with incomplete clinicopathological data, including age, race, primary site, surgical type, and American Joint Committee on Cancer (AJCC) stage.
Covariates
Data available in the SEER database included age (<65 years; ≥65 years), gender (male; female), race (white; black; other), laterality (left; right), differentiation (grade I–II; grade III; grade IV; unknown), AJCC 8th Edition stage group (I; II; III; IV), surgery (no; yes), chemotherapy (no/unknown; yes), and radiation (no/unknown; yes). AJCC 8th edition stage group was calculated for each patient based on tumor size, extension, and 7th edition N/M stage.
The endpoints of this study were overall survival (OS), defined as the time from diagnosis to death from any cause or date of the last follow-up, and cancer-specific survival (CSS), defined as the time from diagnosis to death caused by PSC or date of the most recent follow-up. The SEER 18 Database contains information on deaths up until 2016; therefore, the cut-off date was set at December 31, 2016.
Statistical methods
The Kaplan-Meier survival curves were compared using the log-rank test. A P value of <0.05 was considered statistically significant. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were calculated. The Cox proportional hazards model was used to conduct both univariate and multivariate survival analysis.
Propensity score matching (PSM) analysis (including race, laterality, surgery, chemotherapy, and radiation) was performed to compare OS and CSS in elderly versus non-elderly patients. Propensity matching was performed on a one-to-one basis using nearest neighbor matching without replacement (caliper 0.01).
SPSS software (SPSS Inc., Chicago, IL, USA, version 23) was used for statistical analysis, and GraphPad Prism 8 was used to generate the survival curves. PS-matching package version 3.04 and SPSS statistics R essentials were used for statistical analyses.
Results
Patient characteristics
A total of 1,039 patients diagnosed with PSC between 2004 and 2016 were identified. Patient demographic information is shown in Figure 1. The median age at diagnosis was 68 years (1–94 years), with 618 patients (59.5%) ≥65 years old, and more male than female patients (59.3% vs. 49.7%). High-grade tumors and undifferentiated tumors represented 52.5% and 46.1% of tumor types, respectively. Our cohort comprised spindle cell carcinoma (28.7%), giant cell carcinoma (24.3%), pleomorphic carcinoma (28.4%), carcinosarcoma (16.2%), and pulmonary blastoma (2.4%). Advanced cancer stages (III–IV) were prevalent (74.8%).
Data showed that 396 (38.1%) patients received surgery, 432 (36.8%) patients received chemotherapy, and 139 (13.4%) patients were treated with radiotherapy.
Survival data and related prognostic indicators
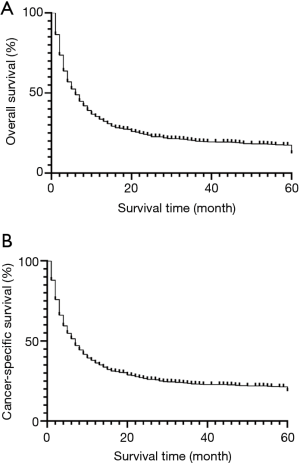
Overall, PSC patients had poor outcomes, with a median OS of 6 months. The 5-year OS and CSS rates were 12.3% and 18.7%, respectively. The OS and CSS curves are shown in Figure 2. In the univariate analyses, age (P<0.001), gender (P=0.001), differentiation (P<0.001), histology (P<0.001), AJCC 8th edition stage groups (P<0.001), surgery (P<0.001), chemotherapy (P<0.001), and radiation (P=0.003) were predictors of OS (Table 1). Multivariate analysis further revealed that age (≥65 years, HR =1.409, P<0.001), histology (giant cell carcinoma, HR =1.264, P<0.037), and advanced AJCC stage (stage III, HR =2.777, P<0.001; stage IV, HR =5.100, P<0.001) were independent, unfavorable prognostic factors, while female sex (HR =0.750, P<0.001), surgery (HR =0.484, P<0.001), chemotherapy (HR =0.504, P<0.001), and radiation (HR =0.801, P<0.001) were independent, favorable prognostic factors.
Full table
Clinicopathological data and survival of the elderly and the non-elderly
We compared clinicopathological parameters and survival data of the elderly (≥65 years) and the non-elderly (<65 years) patients. Basic patient information before matching is shown in Table 2. To reduce selection bias, a PSM analysis was undertaken. A total of 657 patients were successfully matched, 337 patients in the elderly group and 320 patients in the non-elderly group. In the elderly group, the 5-year OS and CSS rates were 9.3% and 15.2%, respectively, compared with 17.3% and 22.3%, respectively in the non-elderly group (Figure 3). Multivariate analysis showed the independent, favorable prognostic factors to be female sex (HR =0.656, P=0.001) and chemotherapy (HR =0.530, P<0.001) in the elderly group, and surgery (HR =0.551, P<0.001) and chemotherapy (HR =0.573, P<0.001) in the non-elderly group (Table 3).
Full table


Full table
Discussion
PSC is an under-researched disease, particularly in the elderly, and patients have a poor prognosis. Histologically, PSC is recognized as a change from the typical epithelioid morphology of carcinoma, to giant cells or spindle-shaped cells that morphologically mimic sarcoma. Uncommonly, true heterologous sarcomatous types, such as rhabdomyoblastic differentiation or malignant cartilage formation, are included. If the area of sarcomatoid change constitutes more than 10% of the tumor, a diagnosis of sarcomatoid carcinoma is given per the current WHO criteria; therefore, it is difficult to provide an accurate diagnosis preoperatively. In most cases, a conclusive pathological diagnosis is established based on a surgically resected specimen (7) (Figure 4). In the WHO classification of lung tumors, these neoplasms have undergone frequent reclassification over the years. However, the conceptual approach proposed by the WHO is not without controversy. The variable use of terminology for these tumors and the inclusion by the WHO has largely complicated the collection of uniform clinical data. In addition, the significance of such histology in terms of treatment and prognosis has been particularly difficult to ascertain. The comprehensive immunohistochemical studies of larger series of these tumors are still lacking.
We found that PSC was more often associated with males (59.3%), which is similar to the findings of Martin et al. (8) (54%), but is far lower than previous reports of over 90% (9,10). PSC is pathologically high-grade, with poor prognosis (11), and these clinicopathological features were confirmed in our study. In addition, our study found that most PSC patients were at an advanced stage when diagnosed. Since PSC is generally a form of high-grade and advanced-stage tumor progression in non-small cell carcinoma, it is unsurprising that it is associated with a poor prognosis (2). The median survival of PSC had been reported to range from 8 to 19 months (12-17), which is inferior to other non-small cell lung carcinomas, and is similar to our findings that prognosis was poor even in patients with early-stage PSCs (stage I/II). The median OS in our study was 36 months.
We compared prognostic factors between elderly and the non-elderly patients. The 5-year OS and CSS rates of the elderly patients were poorer than those of the non-elderly patients. Stage was an independent, unfavorable prognostic factor. Female sex and chemotherapy were protective factors for OS in elderly patients, while surgery and chemotherapy were protective factors in non-elderly patients. It is noted that most elderly patients cannot tolerate surgery or chemotherapy, most likely due to poor physical status and basic diseases.
Primary surgery is the mainstream treatment for early stage patients, with a median survival time of approximately 14 months (18,19). Our research showed that surgery was a positive independent prognostic factor, further confirming its importance in treatment of PSC. The roles of chemotherapy and radiotherapy in PSC treatment are still unclear. While the overall response rate to chemotherapy can be extremely low, ranging from 0 to 17% (20), and irradiation can cause sarcomatous or anaplastic changes in carcinoma (21), our study found that chemotherapy and radiotherapy were independent protective factors in PSC.
New hope for therapy in PSC exists, primarily due to the discovery of the MET exon 14 skipping mutation. MET exon 14 skipping mutations lead to increased signaling through the MET receptor pathway, and patient tumor responses have been noted with targeted MET tyrosine kinase inhibitors (22,23). However, two recent studies found that 30–40% of PSC patients had KRAS mutations, causing poor clinical outcomes (24,25). Unfortunately, it is difficult to treat KRAS mutations with targeted therapy. Another promising therapeutic option in PSC is immune checkpoint inhibitors, as over half of PSC tumors have been shown to have PD-L1 overexpression (26-28).
Although our results may offer physicians a better understanding of the clinicopathological features and survival with PSC, there are limitations to our research. First, SEER does not include some important variables, such as chemotherapy regimens, genomic analyses, and surgical margin status. Second, PSC is a rare tumor, and it is possible that PSC was incorrectly diagnosed in some cases. Finally, selection bias may exist because there are inherent confounding effects.
Ultimately, our study showed that PSC has a higher incidence in the elderly, in males, and in high-grade and advanced AJCC stage. Female sex, surgery, chemotherapy, and radiation are independent protective factors of OS. Elderly patients are associated with worse outcomes than non-elderly patients. However, based on an understanding of its molecular biology, broad immunologic and molecular testing may be used to direct therapeutic options.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-6213
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6213). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Yendamuri S, Caty L, Pine M. Outcomes of sarcomatoid carcinoma of the lung: a surveillance, epidemiology, and end results database analysis. Surgery 2012;152:397-402. [Crossref] [PubMed]
- Shokralla HA, Rahouma M. Prognostic clinico-pathological features of 99 cases advanced non-small cell lung cancer—Egyptian National Cancer Institute. Adv Lung Cancer 2016;4:29. [Crossref]
- Vokes EE, Chu E. Anti-EGFR therapies: clinical experience in colorectal, lung, and head and neck cancers. Oncology (Williston Park) 2006;20:15-25. [PubMed]
- Stiles BM, Nasar A, Hussein MK, Ghaly GR, Ahmed MR, Port JL, et al. Routine molecular testing of resected early-stage lung adenocarcinoma with targeted next-generation sequencing demonstrates a high rate of actionable mutations. J Thorac Oncol 2016;11:S44-5. [Crossref]
- Duggan MA, Anderson WF, Altekruse S. The surveillance, epidemiology, and end results (SEER) program and pathology: toward strengthening the critical relationship. Am J Surg Pathol 2016;40:e94-102. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus, and Heart. In: FT Bosman, ES Jaffe, SR Lakhani, et al. editors. World Health Organization Classification of Tumours, Lyon: IARC Press, 2015.
- Martin LW, Correa AM, Ordonez NG, et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg 2007;84:973-80. [Crossref] [PubMed]
- Chang YL, Lee YC, Shih JY. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer 2001;34:91-7. [Crossref] [PubMed]
- Rossi G, Cavazza A, Sturm N, et al. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol 2003;27:311-24. [Crossref] [PubMed]
- Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol 2010;18:103-20. [Crossref] [PubMed]
- Pelosi G, Gasparini P, Cavazza A, et al. Multiparametric molecular characterization of pulmonary sarco-matoid carcinoma reveals a nonrandom amplification of anaplastic lymphoma kinase (ALK) gene. Lung Cancer 2012;77:507-14. [Crossref] [PubMed]
- Nappi O, Glasner SD, Swanson PE. Biphasic and monophasic sarcomatoid carcinomas of the lung: a reappraisal of “carcinosarcomas” and “spindle-cell carcinomas”. Am J Clin Pathol 1994;102:331-40. [Crossref] [PubMed]
- Nishida K, Kobayashi Y, Ishikawa Y, et al. Sarcomatoid adenocarcinoma of the lung: clinicopathological, immunohistochemical and molecular analyses. Anticancer Res 2002;22:3477-83. [PubMed]
- Raveglia F, Mezzetti M, Panigalli T, et al. Personal experience in surgical management of pulmonary pleomorphic carcinoma. Ann Thorac Surg 2004;78:1742-7. [Crossref] [PubMed]
- Ro JY, Chen JL, Lee JS. Sarcomatoid carcinoma of the lung. Immunohistochemical and ultrastructural studies of 14 cases. Cancer 1992;69:376-86. [Crossref] [PubMed]
- Hummel P, Cangiarella JF, Cohen JM. Transthoracic fine-needle aspiration biopsy of pulmonary spindle cell and mesenchymal lesions. Cancer 2001;93:187-98. [Crossref] [PubMed]
- Shum E, Stuart M, Borczuk A, et al. Recent advance in management of pulmonary sarcomatoid carcinoma. Expert Rev Respir Med 2016;10:407-16. [Crossref] [PubMed]
- Petrov DB, Vlassov VI, Kalaydjiev GT, et al. Primary pulmonary sarcomas and carcinosarcomas-postoperative results and comparative survival analysis. Eur J Cardiothorac Surg 2003;23:461-6. [Crossref] [PubMed]
- Ouziane I, Boutayeb S, Mrabti H, et al. Sarcomatoid carcinoma of the lung: a model of resistance of chemotherapy. N Am J Med Sci 2014;6:342-5. [Crossref] [PubMed]
- Steuer CE, Behera M, Liu Y, et al. Pulmonary sarcomatoid carcinoma: an analysis of the National Cancer Data Base. Clin Lung Cancer 2017;18:286-92. [Crossref] [PubMed]
- Lee C, Usenko D, Frampton GM. MET 14 Deletion in Sarcomatoid Non-Small-Cell Lung Cancer Detected by Next-Generation Sequencing and Successfully Treated with a MET Inhibitor. J Thorac Oncol 2015;10:e113-4. [Crossref] [PubMed]
- Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015;5:842-9. [Crossref] [PubMed]
- Lococo F, Gandolfi G, Rossi G, et al. Deep Sequencing Analysis Reveals That KRAS Mutation Is a Marker of Poor Prognosis in Patients with Pulmonary Sarcomatoid Carcinoma. J Thorac Oncol 2016;11:1282-92. [Crossref] [PubMed]
- Terra SB, Jang JS, Bi L, et al. Molecular characterization of pulmonary sarcomatoid carcinoma: analysis of 33 cases. Mod Pathol 2016;29:824-31. [Crossref] [PubMed]
- Vieira T, Antoine M, Hamard C, et al. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) and strong immune-cell infiltration by TCD3 cells and macrophages. Lung Cancer 2016;98:51-8. [Crossref] [PubMed]
- Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol 2013;8:803-5. [Crossref] [PubMed]
- Kim S, Kim MY, Koh J, et al. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: Comparison of sarcomatous and carcinomatous areas. Eur J Cancer 2015;51:2698-707. [Crossref] [PubMed]



