
Exosomal microRNA-155 as a biomarker for hepatic fibrosis diagnosis and progression
Introduction
The activation of hepatic stellate cells (HSCs) results in hepatic fibrosis (1-3). Liver fibrosis is closely associated with the development of hepatocellular carcinoma (HCC); therefore, the accurate assessment of fibrosis is critical for HCC screening (4). Distinguishing patients with advanced disease, especially cirrhosis, from patients with mild or no fibrosis is important for clinical treatment decisions (5).
Exosomes are small extracellular membrane vesicles (EVs) containing nucleic acids and proteins excreted by HSCs and mesenchymal stem cells (6,7). Damaged epithelial cells secrete exosomes that activate fibrocytes (8). In addition to exosomes, there are other EVs including microvesicles and apoptotic bodies, EVs enter the intercellular space or they may enter body fluids such as urine, saliva or blood, which may be taken up by neighbouring cells or potentially be delivered to target cells. Exosomes are a readily accessible and rich source of biomarkers that have potential for assessing organ disease, including that of the liver (9,10). miRNAs, 19–24 nucleotide non-coding RNAs, negatively regulate gene expression and profibrogenic signaling (11,12). miRNAs are involved in HSC activation, and regulate apoptosis, differentiation, migration, and proliferation of activated HSCs (13,14). Circulating miRNAs are associated with the progression of fibrosis, and may be promising fibrotic markers present in plasma (15,16). However, the clinical benefit of using exosomal miRNAs for assessing the stage of hepatic fibrosis is not yet clear. Several studies have shown that miRNA-155 (miR-155) is closely associated with hepatic cancer and hepatitis (17-19). miR-155 appeared in different serum components in different liver injuries. In alcohol liver injury, miR-155 was found in the EV-enriched serum fraction, whereas in APAP-induced DILI, miR-155 is mainly present in the protein rich fraction (20). Therefore, miR-155 in different fractions of serum may be a potential biomarker for identifying the aetiology of liver injury, and it will be interesting to further explore its contribution to communication between hepatic fibrosis and hepatic cancer. profiling exosomal miR-155 during the progression of fibrosis may identify new diagnostic biomarkers and therapeutic targets for the treatment of hepatic fibrosis.
Pathological examination of liver biopsies remains the gold standard for evaluating the stage of hepatic fibrosis (21,22). There are a number of disadvantages associated with biopsy; for example, sampling errors and intra- and inter-observer variations (23,24). In addition to pathological examination to stage liver fibrosis, albumin, Type IV, hydroxyproline (Hyp), aspartate aminotransferase (AST), aminotransferase (ALT), albumin, hemoglobin, bilirubin ascites and other indicators can indirectly reflect the degree of liver fibrosis. In this context, the inclusion of exosomal miRNAs in the prediction algorithms for determining hepatic fibrosis stage may be useful, although this has not yet been investigated. In the present study, the capacity of exosomal miR-155 as a non-invasive biomarker to predict the stage of hepatic fibrosis in patients was investigated.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-20-7787).
Methods
Plasma sampling
Peripheral blood serum was collected from each participant (50 healthy volunteers and 94 patients with cirrhosis) and liver fibrosis model rats and transferred to tubes. The tubes were centrifuged at 1,200 ×g for 15 min at 26 °C. After plasma centrifugation, the samples were stored in liquid nitrogen until use. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Tianjin First Central Hospital authorized the study protocol. All participants provided written informed consent ahead of recruitment.
Animal model
All animal procedures followed national guidelines for animal care and use, and were authorized by the Animal Care Ethics Committee of Tianjin First Central Hospital. Adult male Sprague-Dawley rats, weighing 200–220 g and raised on standard laboratory chow with freely available drinking water, were used in the experiments. They were housed at 23 °C with a light/dark (12 h/12 h) cycle. A total of 56 rats were randomly assigned to 7 groups (n=8, assigned as 0, 2, 4, 6, 8, 10, and 12 weeks). Rats in all groups were intravenously injected with CCl4 (3% vol/vol in olive oil; 0.3 mL/100 g body weight) twice weekly. When the liver and blood were harvested, the rats were killed by cervical dislocation under anesthesia. Blood was collected from the vena cava at each time point, and the plasma was collected according to the procedure described above. A portion of each rat liver was soaked in formalin, and the remainder was stored in liquid nitrogen for later use.
Exosome extraction and identification
Exosomes were isolated from plasma samples using the Exoquick kit (System Biosciences, California, USA) according to the manufacturer’s protocol. Briefly, 0.4 mL plasma and 0.1 mL Exoquick kit were mixed and incubated for 12 h at 4 °C, and then centrifuged at room temperature at 10,000 ×g for 30 min. Exosomes were used for RNA extraction and characterization.
Transmission electron microscopy (TEM) to verify exosome morphology
TEM (JEM-2100; Jeol, Tokyo, Japan) was used to observe the extracted pellets and to validate characterization. Purified exosomes (10 µL) were fixed with 1% glutaraldehyde for 10 min, washed, and contrasted in 2% uranyl acetate.
Western blot analysis of CD63 and CD9
Proteins extracted from plasma exosomes were loaded onto 10% gels and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred to polyvinylidene difluoride membranes (EMD Millipore, Temecula, CA, USA). The membranes were probed with primary antibodies at 4 °C for ~12 h and then with secondary antibodies for 1 h. The signal was developed using enhanced chemiluminescence reagent (EMD Millipore, Temecula, CA, USA), and visualized with the FluorChem FC2 Imaging System (Alpha Innotech, California, USA). Western blot analysis was performed following a standard protocol using antibodies targeting CD9 and CD63 (dilution 1:200; Wanleibio, Shenyang, China).
Quantification of miR-155 mRNA expression by quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from exosomes. To validate miRNA expression, qRT-PCR was performed using a SYBR Premix Dimereraser kit (TaKaRa Biotechnology, Kyushu, Japan) on a LightCycler 480 II detection system (Roche Diagnostics, New Naxi, USA). The primers for miR-155 and U6 were purchased from Sangon Biotech (Shanghai, China). The primers are listed in Table 1. The relative gene expression values for the target miRNA were calculated using the 2–ΔΔCT method.

Full table
Hematoxylin-eosin (HE) staining and histopathological observation
Sixty 3-µm sections were obtained from each paraffin block using a microtome (RM2255; Leica, Frankfurt, Germany) and stained with HE. Samples were immersed in xylene and alcohol, stained with hematoxylin for 5 min, stained with eosin for 3 min, and re-immersed in alcohol and xylene. Slides were mounted using a synthetic resin (Entellan; Merck, Darmstadt, Germany). Paraffin sections of liver tissue were prepared for histopathological observation, and the degree of hepatic necrosis and fibrosis was observed by HE staining.
Plasma measurements
Type IV collagen (CIV), hydroxyproline (Hyp), aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, hemoglobin, and platelets in plasma samples were measured at the Tianjin First Center Hospital Laboratory.
Statistical analysis
Statistical analysis was performed using SPSS version 24.0 (IBM, Armonk, NY, USA). The results are presented as means ± standard deviations. Two groups were compared using a t-test. Independent risk factors of relationships between different groups in the in vitro assay data were analyzed using analysis of variance. Receiver-operating characteristic (ROC) curve analysis was conducted to determine the accuracy of the diagnosis of hepatic fibrosis. P<0.05 was considered to indicate a statistically significant difference.
Results
Characterization of isolated exosomes
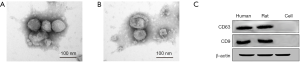
Exosomes were characterized by western blot analysis and TEM to ensure the quality and efficiency of the exosomes (Figure 1). Exosome analysis by electron microscopy was consistent with previous reports (Figure 1A,B). Furthermore, western blot analysis of CD9 and CD63 were used to confirm the presence of exosomes (Figure 1C). The successful isolation of exosomes was confirmed by these results.
Clinical significance of exosomal miR-155 for liver fibrosis staging
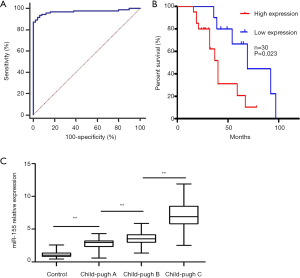
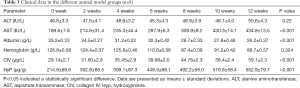
To better understand the potential roles of plasma exosomal miR-155 in liver fibrosis development and progression, the clinical significance of miR-155 expression in the plasma exosomes of the 94 patients with liver fibrosis and 50 healthy individuals was validated by qRT-PCR. The Child-Pugh rating was used to categorize the 94 liver fibrosis cases into 3 groups. As expected, there was a significant correlation between miR-155 expression and Child-Pugh score (r2=0.728, P<0.01). Additionally, miR-155 expression in patients with a Child-Pugh C rating was substantially higher than in patients with a Child-Pugh A and B rating (Figure 2A). The ROC curve [area under the curve (AUC): 0.971, sensitivity: 93.62%, specificity: 94%] showed that exosomal miR-155 may have diagnostic value for liver fibrosis (Figure 2B). Furthermore, the association between plasma exosomal miR-155 expression and the prognosis of 30 patients with primary liver fibrosis who underwent liver transplantation was examined. Further research suggested that the low miR-155 expression group had a significantly better prognosis than the high expression group (P=0.016) (Figure 2C). Plasma analysis also revealed that there were differences in AST (P<0.001), albumin (P<0.001), platelet count (P<0.001), and age (P=0.028) between the Child-Pugh C group and the Child-Pugh A and B group. However, the Child-Pugh score was not associated with several other clinical features, including sex, ALT, and hemoglobin (Table 2). Therefore, we hypothesized that miR-155 expression can predict the stage of liver fibrosis. To validate our hypothesis, we produced rat liver fibrosis models of different stages, as described earlier.
Full table
Results of liver fibrosis rat models
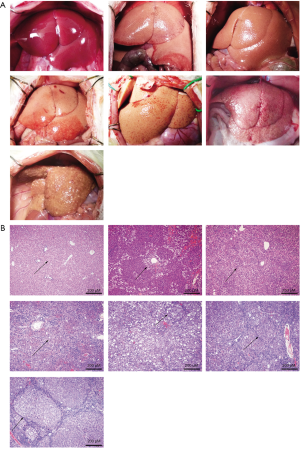
As described earlier, a rat liver fibrosis model was established. The prolongation of CCl4 treatment significantly increased the degree of hepatic necrosis and cirrhosis. Hepatic fibrosis gradually worsened over time, and from week 10 onwards, a marked reduction in hepatic volume was observed. HE staining revealed that hepatic necrosis and hepatic fibrosis gradually worsened. These results demonstrated that the fibrosis model had been successfully established with different degrees of liver necrosis and cirrhosis (Figure 3).
Exosomal miR-155 and clinical indicators of different degrees of hepatic necrosis and fibrosis
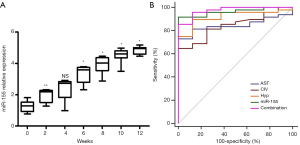
In the present study, we found that, with the increase in hepatic necrosis and hepatic fibrosis, the expression of exosomal miR-155 significantly increased relative to the control group (P<0.05) (Figure 4A). The results revealed that AST, albumin, CIV, and Hyp were significantly associated with hepatic fibrosis (P<0.05); however, other clinical features, including ALT and hemoglobin, were not associated with fibrosis (Table 3). The ROC curve showed that exosomal miR-155 has diagnostic value for liver fibrosis, more so than CIV, Hyp, or AST. Combined diagnosis using miR-155, CIV, Hyp, and AST had the highest AUC value of 0.974 (sensitivity: 85.4% and specificity: 98.7%) (Figure 4B).
Full table
Discussion
The activation of hepatic stellate cells (HSCs) results in hepatic fibrosis. Liver fibrosis is closely associated with the development of HCC, so the accurate assessment of fibrosis is critical for HCC screening (4,25,26). A number of studies have found that HSC-derived exosomes promote fibrosis. The activated HSCs can release exosomes containing CCN2, which may amplify fibrogenic signalling to promote hepatic fibrosis (27). Kostallari et al. reported that the PDGF receptor-alpha (PDGFRα)-enriched exosomes released by PDGF-BB–treated HSCs promote HSC migration and liver fibrosis (28). MiRNAs have the potential to become novel, non-invasive biomarkers because they are highly stable and easily detected in circulation. Indeed, numerous studies have shown that several miRNAs have certain advantages as biomarkers for early diagnosis, prognosis and evaluation of hepatic fibrosis compared with traditional biomarkers. In addition, exosomes derived from HSCs contribute to the pathology of liver cancer. Researchers showed that exosomes derived from HSCs deliver miR-335-5p to recipient hepatocellular carcinoma (HCC) cells, inhibiting the proliferation and in vitro invasion of HCC cells and inducing the shrinkage of HCC tumours in vivo (29). Moreover, researchers observed that the expression of miR-30a was down-regulated in exosomes derived from activated HSCs, which may prevent HSC activation by suppressing autophagy (30).
miR-155 has various liver-associated roles, including effects on hepatic cancer, hepatic injury, hepatic fibrosis, and lipid metabolism (17,31,32). It was demonstrated that miR-155, a pro-fibrotic cytokine positively regulated by hypoxia inducible factor-1α (HIF-1α), adjusted both transforming growth factor-β1 and the process of epithelial-mesenchymal transition (EMT) under hypoxia to promote fibrosis of proximal tubule cells. Another study showed that hsa-miR-155-5p was correlated with hepatic inflammatory level and fibrosis (33). In our study, miR-155 was also significantly upregulated in Child-Pugh C, and demonstrated excellent diagnostic value for advanced liver fibrosis. At the same time, the expression level of miR-155 may be used to predict the clinical outcome of liver transplantation patients; patients with high expression are likely to have reduced survival time after liver transplantation compared with patients with low expression. miR-155 is closely related to the progression of cirrhosis and clinical prognostic indicators of cirrhosis. Furthermore, exosomal miR-155 gradually increased with the degree of hepatic necrosis and liver fibrosis induced by CCl4. Combined diagnosis with miR-155, CIV, Hyp, and AST had the highest AUC, indicating that the diagnostic accuracy was improved. Exosomal microRNA-155 can be used as a non-invasive marker to avoid complications caused by liver biopsy. It has the potential to reflect these dynamic changes, which could help in identifying patients at risk for progressive fibrosis to allow for earlier intervention or maintain closer surveillance. However, there is a lack of relevant long-term longitudinal and clinical data about microRNA-155, so it is difficult to apply to clinic in the short term.
In conclusion, exosomal miR-155 was identified to be associated with liver fibrosis, according to the results obtained in the present study. It had a significant correlation with liver fibrosis, and may therefore serve as a sensitive biomarker for patients with liver fibrosis.
Acknowledgments
Funding: The present study was supported by the Science and Technology Fund of Tianjin Health Bureau (No. 2014KR08) and Key Projects of Health Industry in Tianjin (No. 16KG108).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-7787
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-7787
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-7787). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Tianjin First Central Hospital authorized the study protocol. All participants provided written informed consent ahead of recruitment. All animal procedures followed national guidelines for animal care and use, and were authorized by the Animal Care Ethics Committee of Tianjin First Central Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Feili X, Wu S, Ye W, et al. MicroRNA-34a-5p inhibits liver fibrosis by regulating TGF-β1/Smad3 pathway in hepatic stellate cells. Cell Biol Int 2018;42:1370-6. [Crossref] [PubMed]
- Tee JK, Peng F, Tan YL, et al. Magnesium Isoglycyrrhizinate Ameliorates Fibrosis and Disrupts TGF-β-Mediated SMAD Pathway in Activated Hepatic Stellate Cell Line LX2. Front Pharmacol 2018;9:1018. [Crossref] [PubMed]
- Morsy MA, Nair AB. Prevention of rat liver fibrosis by selective targeting of hepatic stellate cells using hesperidin carriers. Int J Pharm 2018;552:241-50. [Crossref] [PubMed]
- Toyoda H, Kumada T, Tada T, et al. Impact of hepatocellular carcinoma etiology and liver function on the benefit of surveillance: a novel approach for the adjustment of lead-time bias. Liver Int 2018;38:2260-8. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol 2018;69:461-511. [Crossref]
- Thery C, Amigorena S, Raposo G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006;Chapter 3:Unit 3.22.
- Kalimuthu S, Gangadaran P, Rajendran RL, et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol 2018;9:1116. [Crossref] [PubMed]
- Borges FT, Melo SA, Ozdemir BC, et al. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 2013;24:385-92. [Crossref] [PubMed]
- Leszczynska A, Kulkarni M, Ljubimov AV, et al. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci Rep 2018;8:15173. [Crossref] [PubMed]
- Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 2015;6:127. [Crossref] [PubMed]
- de Oliveira da Silva B, Alberici LC, Ramos LF, et al. Altered global microRNA expression in hepatic stellate cells LX-2 by angiotensin-(1-7) and miRNA-1914-5p identification as regulator of pro-fibrogenic elements and lipid metabolism. Int J Biochem Cell Biol 2018;98:137-55. [Crossref] [PubMed]
- El-Wakeel SA, Rahmo RM, El-Abhar HS. Anti-fibrotic impact of Carvedilol in a CCl-4 model of liver fibrosis via serum microRNA-200a/SMAD7 enhancement to bridle TGF-beta1/EMT track. Sci Rep 2018;8:14327. [Crossref] [PubMed]
- Chen L, Chen R, Kemper S, et al. Therapeutic effects of serum extracellular vesicles in liver fibrosis. J Extracell Vesicles 2018;7:1461505. [Crossref] [PubMed]
- You K, Li SY, Gong J, et al. MicroRNA-125b Promotes Hepatic Stellate Cell Activation and Liver Fibrosis by Activating RhoA Signaling. Mol Ther Nucleic Acids 2018;12:57-66. [Crossref] [PubMed]
- El-Ahwany E, Nagy F, Zoheiry M, et al. Circulating miRNAs as Predictor Markers for Activation of Hepatic Stellate Cells and Progression of HCV-Induced Liver Fibrosis. Electron Physician 2016;8:1804-10. [Crossref] [PubMed]
- Matsuura K, De Giorgi V, Schechterly C, et al. Circulating let-7 levels in plasma and extracellular vesicles correlate with hepatic fibrosis progression in chronic hepatitis C. Hepatology 2016;64:732-45. [Crossref] [PubMed]
- Bala S, Csak T, Saha B, et al. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J Hepatol 2016;64:1378-87. [Crossref] [PubMed]
- Hyun J, Park J, Wang S, et al. MicroRNA Expression Profiling in CCl(4)-Induced Liver Fibrosis of Mus musculus. Int J Mol Sci 2016;17:961. [Crossref] [PubMed]
- Fu X, Wen H, Jing L, et al. MicroRNA-155-5p promotes hepatocellular carcinoma progression by suppressing PTEN through the PI3K/Akt pathway. Cancer Sci 2017;108:620-31. [Crossref] [PubMed]
- Bala S, Petrasek J, Mundkur S, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 2012;56:1946-57. [Crossref] [PubMed]
- Pinzani M, Rombouts K, Colagrande S. Fibrosis in chronic liver diseases: diagnosis and management. J Hepatol 2005;42 Suppl:S22-36. [Crossref] [PubMed]
- Palacios Pérez A, Salmeron EJ. Role of liver biopsy in the diagnosis and management of chronic hepatitis C. Gastroenterol Hepatol 2007;30:402-7. [PubMed]
- Maruyama H, Shiha G, Yokosuka O, et al. Non-invasive assessment of portal hypertension and liver fibrosis using contrast-enhanced ultrasonography. Hepatol Int 2016;10:267-76. [Crossref] [PubMed]
- Li C, Li R, Zhang W. Progress in non-invasive detection of liver fibrosis. Cancer Biol Med 2018;15:124-36. [Crossref] [PubMed]
- Koda M, Tanaka S, Takemura S, et al. Long-term prognostic factors after hepatic resection for hepatitis C virus-related hepatocellular carcinoma, with a special reference to viral status. Liver Cancer 2018;7:261-76. [Crossref] [PubMed]
- Ishaque T, Massie AB, Bowring MG, et al. Liver Transplantation and Waitlist Mortality for HCC and Non-HCC Candidates Following the 2015 HCC Exception Policy Change. Am J Transplant 2019;19:564-72. [Crossref] [PubMed]
- Sancho-Bru P, Altamirano J, Rodrigo-Torres D, et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology 2012;55:1931-41. [Crossref] [PubMed]
- Kostallari E, Hirsova P, Prasnicka A, et al. Hepatic stellate cell-derived platelet-derived growth factor receptor-alpha-enriched extracellular vesicles promote liver fibrosis in mice through SHP2. Hepatology 2018;68:333-48. [Crossref] [PubMed]
- Wang F, Li L, Piontek K, et al. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology 2018;67:940-54. [Crossref] [PubMed]
- Chen J, Yu Y, Li S, et al. MicroRNA-30a ameliorates hepatic fibrosis by inhibiting Beclin1-mediated autophagy. J Cell Mol Med 2017;21:3679-92. [Crossref] [PubMed]
- Tang B, Lei B, Qi G, et al. MicroRNA-155-3p promotes hepatocellular carcinoma formation by suppressing FBXW7 expression. J Exp Clin Cancer Res 2016;35:93. [Crossref] [PubMed]
- Lin X, Jia J, Du T, et al. Overexpression of miR-155 in the liver of transgenic mice alters the expression profiling of hepatic genes associated with lipid metabolism. PLoS One 2015;10:e0118417. [Crossref] [PubMed]
- Abebayehu D, Spence AJ, Qayum AA, et al. Lactic Acid Suppresses IL-33-Mediated Mast Cell Inflammatory Responses via Hypoxia-Inducible Factor-1alpha-Dependent miR-155 Suppression. J Immunol 2016;197:2909-17. [Crossref] [PubMed]
(English Language Editor: R. Scott)


