
A nomogram for predicting the risk of no-reflow after primary percutaneous coronary intervention in elderly patients with ST-segment elevation myocardial infarction
Introduction
Cardiovascular diseases (CVDs) have become the focus of global public health problems and the greatest threat to human health (1). In recent years, the morbidity and mortality of CVDs have been increasing with years, and CVDs are also the first cause of death among urban and rural residents in China (2). Acute myocardial infarction (AMI) is the most critical disease in CVDs, with a high mortality and disability rate (3). Recently, it showed an increasing incidence trend of acute ST-segment elevation myocardial infarction (STEMI). And with the accelerating aging of the population, the proportion of elderly STEMI patients (over 65 years old) is increasing as well. It has been reported that STEMI is a severe and fatal disease in the elderly, which affects the life safety of the elderly (4,5). There are more patients with ischemic cardiomyopathy due to low cardiac contractility reserve in elderly patients. Once the STEMI occurs, it is prone to induce severe hemodynamic disorders, including acute pulmonary edema and cardiogenic shock, and the long-term prognosis is poor. In addition, the risk factors of STEMI include the increase of age and heart rate, the decrease of systolic blood pressure, blood glucose, dyslipidemia and the history of coronary heart disease. Therefore, the application of proper treatment methods is another crucial factor in improving the prognosis of STEMI. Early implementation of treatment is required. The implementation of effective early reperfusion therapy to rescue dying myocardium is the critical link of STEMI treatment, which can effectively improve the survival rate and prognosis of patients (6). Emergency percutaneous coronary intervention (PCI) can be used as the most effective method of reperfusion therapy. Studies have shown the application of emergency PCI can continuously and effectively open the infarct-related arteries as early as possible, therefore achieve effective reperfusion of the myocardium, and reduce the fatality rate in STEMI (7,8).
At present, although PCI has been widely used in the clinical treatment of STEMI and has achieved a satisfactory clinical efficacy, no-reflow was still found in some patients after PCI, leading to cardiac dysfunction, left ventricular remodeling, sudden cardiac death, and other complications (9,10). The no-reflow phenomenon refers to the fact that after emergency PCI treatment, although the infarction relevant arteries (IRA) of patients have been opened, there is still no myocardial perfusion or low perfusion (11,12). The No-reflow phenomenon is a severe complication of emergency PCI; the incidence rate is as high as 25–30% (13). The rapid recovery of IRA blood flow is related to the mortality of STEMI patients, and the no-reflow will offset the benefits of IRA recanalization, leading to a poor prognosis (14). Therefore, it is of considerable clinical significance to evaluate the risk of no-reflow before the operation, to identify and screen high-risk no-reflow patients, especially in elderly patients, and to actively implement treatment strategies to prevent no-reflow. However, at present, there are few studies on the risk factor model of no-reflow in elderly STEMI patients who receive PCI treatment.
By retrospectively analyzing the case data of elderly STEMI patients who underwent direct PCI, the purpose of this study was to screen out clinical risk factors related to the occurrence of no-reflow after PCI, construct a nomogram model, and perform relevant evaluation and verification. It can provide some guidance for clinical screening of high-risk patients with no-reflow, reducing the occurrence of related complications, and improving the prognosis of patients. We present the following article in accordance with the TRIPOD reporting checklist (available at http://dx.doi.org/10.21037/atm-20-8003).
Methods
Patients collection
From January 2010 to May 2016, all aspects of clinical information of patients were collected retrospectively, with aged >65 years and underwent direct PCI in the Department of Cardiology of Tianjin Chest Hospital. The diagnosis of STEMI was following the Chinese 2015 Guidelines for the Management of Acute ST-segment Elevation Myocardial Infarction.
The inclusion criteria were: (I) the patient developed ischemic chest pain lasting more than 30 minutes (or comparable symptoms including acute left heart failure, acute gastrointestinal symptoms, and syncope) within 24 hours before admission and was not relieved by taking nitroglycerin and other nitrate drugs; (II) electrocardiogram (ECG) showed ST-segment elevation in at least two adjacent leads; (III) the serum biochemical marker troponin I (TNI) was positively elevated (>1 ng/mL) within 24 hours of the onset of symptoms; (IV) patients aged >65 years.
The exclusion criteria included: (I) patients aged ≤65 years; (II) the onset time more than 24 hours; (III) patients with failed patency of culprit’s vessels; (IV) patients with incomplete clinical data; (V) patients with factors affecting ST-segment changes in ECG; (VI) patients with a malignant tumor or an autoimmune disease.
Finally, 551 elderly STEMI patients (age >65) received direct PCI were enrolled, and then they were randomly divided into a training group (n=386) and a validation group (n=165) according to the ratio of 7:3. Patients in each group were assigned to a no-reflow group and normal blood flow group according to whether there was a no-reflow phenomenon. The clinical information collected included the necessary demographic, clinical, coronary angiography, ECG characteristics, and biochemical indicators (Table S1).
This work was conducted following the Declaration of Helsinki (2013) of the World Medical Association. The study was approved by ethics board of Tianjin Chest Hospital (No: 2020YS-040-01). Because the study was a retrospective cohort study, informed consent was abandoned.
Screen risk factors and construction of the nomogram model
The clinical candidate indexes related to no-reflow were screened out by univariate logistic regression analysis on training group through the generalized linear model (glm) function in R software (version 3.6.3), and the screening criterion was P value <0.05. Then, multivariate logistic backward stepwise regression (likelihood ratio) analysis in SPSS 23.0 software (SPSS, Inc., Chicago, IL, USA) was performed to further screen out the significant risk factors for no-reflow on a criterion of P value <0.05. A nomogram model was built on the strength of the results of multivariate analysis in the training group by the package of rms (version 6.0-0) in R (version 3.6.3).
Evaluation of the screened risk factors
The Chisq test method in the R language (version 3.6.3) was used to calculate the chi-square (χ2) and the degree of freedom (df) of the risk factors used to construct the nomogram model, and then the importance evaluation chart of risk factor was drawn using the ggplot2 package (version 3.3.1). The corrplot package (version 0.84) was used to plot the correlation diagram of risk factors.
Assessment of the performance of the nomogram model
To assess the discriminative ability of the nomogram model, the area under the receiver operating characteristic (ROC) curve (AUC) was estimated. The ROC curve was drawn using the pROC package (version 1.16.2) in the R software (version 3.6.3). The calibration curves of the nomogram-predicted and the actual probability of no-flow were drawn through bootstrapping using 1,000 resampling procedures. Hosmer-Lemeshow test was conducted using the ResourceSelection package (version 0.3-5) in R software (version 3.6.3), to establish the goodness of fit of the nomogram model.
Validation of the nomogram model
Regarding discrimination and calibration, the performance of the model was verified in the validation group and the entire cohort, using the same methods described above.
Clinical availability of the nomogram model
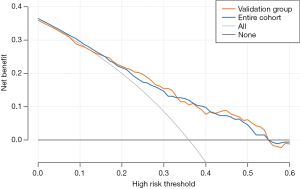
Decision curve analysis (DCA), drawn using the rmda package (version 1.6) in the R software (version 3.6.3), was carried out to evaluate the clinical usefulness of the constructed nomogram by assessing net benefits at various threshold probabilities. Threshold probability stood for the most beneficial region for predicting no-reflow by the nomogram model.
Statistical analysis
The R software (version 3.6.3) was applied for statistical analysis of the clinical data. The index of the measurement type was expressed as the mean ± standard deviation (SD), in which the data conforming to normal distribution were tested by unpaired t-test, and the Mann-Whitney U test tested the non-normal distribution data. Count and percentage expressed the categorical indexes and statistically analyzed by the Chi-square test or Fisher’s exact probability method. Continuous variables were analyzed using the Wilcoxon rank-sum test in an independent sample comparison. For each analysis, the result was statistically significant when P value was less than 0.05.
Results
Clinical characteristics of patients
The clinical information of elderly STEMI patients in the training group and validation group were summed up in Table S1. There were no remarkable differences in any clinical indicators between the two groups (P>0.05, Table S1). There were 245 (245/386, 63.47%) and 105 (105/165, 63.63%) patients with normal blood flow in training and validation groups, respectively. No-reflow happened in 141 (141/386, 36.53%) patients of the training group and 60 (60/165, 36.36%) patients of the validation group. The no-reflow rate did not present a notable difference between the two groups (P=1.000).
Screen risk factors of nomogram model
In training group, after the univariate logistic regression analysis, 22 indexes with P value <0.05, including gender, history of angina pectoris, collateral circulation, and thrombus burden score and so on, were retained and entered into the subsequent multivariate logistic backward stepwise regression (likelihood ratio) analysis (Table 1). The multivariable analyses certified that the occurrence of no-reflow was significantly associated with preoperative TIMI blood flow ≤ grade 1 [odds ratio (OR) 1.939, 95% CI: 1.101–3.415, P=0.022], diameter of target lesion ≥3.5 mm (2.246, 1.332–3.788, P=0.002), collateral circulation (grade 0: 6.09, 0.672–55.229; grade 1: 2.224, 0.222–22.245; grade 2: 2.003, 0.172–23.362, P=0.009), pulse pressure (0.98, 0.967–0.994, P=0.004) and the number of leads for ST segment elevation (1.297, 1.073–1.566, P=0.007, Table 2).
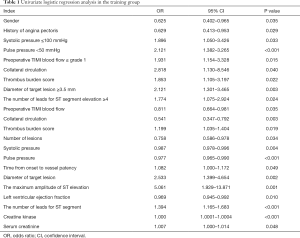
Full table
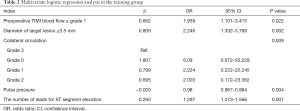
Full table
Evaluation of the screened risk factors
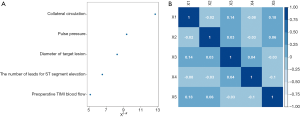
The importance of each screened risk factor was estimated by the partial chi-square statistic minus the predicted degrees of freedom (χ2 − df, Figure 1A). The collateral circulation (χ2 − df =12.70) was the most critical risk factor, followed by pulse pressure (9.37) and diameter of the target lesion (8.26) etc. We also further analyzed the pairwise relationship between the screened risk factors. It could be observed that preoperative TIMI blood flow and the number of leads for ST-segment elevation had the most significant correlation (correlation coefficient: 0.18, Figure 1B), which was preoperative TIMI blood flow and collateral circulation (0.14, Figure 1B) and so on.
Construction of the nomogram model
According to the multivariate regression analysis, a nomogram incorporating the five significant risk factors was developed for predicting no-reflow (Figure 2A). Additionally, point assignments and predictive scores of each factor in the nomogram were listed in Table 3. From the nomogram, the score of preoperative TIMI blood flow ≤ grade 1 was 28, the diameter of the target lesion ≥3.5 mm was 34. With the decrease of collateral circulation grade and pulse pressure, the corresponding scores of these two factors showed an increasing trend. However, the score of the number of leads for ST-segment elevation showed an opposite trend, that is, as the number of leads for ST-segment elevation increased, the corresponding score also increased (Figure 2A). The higher the score, the higher the probability of no-reflow after PCI.


Full table
Assessment of the nomogram model performance
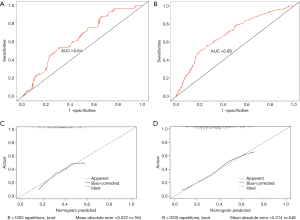
In the training group, the ROC showed that the nomogram model had substantial discrimination (Figure 2B), with an AUC of 0.71 (95% CI: 0.66–0.77). The Hosmer-Lemeshow test obtained a value of P=0.61, which was not statistically significant, showing it did not deviate from the perfect fit. Furthermore, Figure 2C presented the calibration curve of the proposed nomogram, which illustrated the probabilities of no-flow predicted by the nomogram were in good agreement with the actual probabilities.
Validation of the nomogram model
The AUC values of the nomogram were 0.64 (95% CI: 0.56–0.73) and 0.69 ((95% CI: 0.65–0.74) in the validation group and entire cohort, respectively (Figure 3A,B). In the two groups, the Hosmer-Lemeshow test also showed there was no statistical significance (P=0.23 and P=0.50, respectively). Precise calibrations were observed for the probability of non-reflow in the validation group and the entire cohort (Figure 3C,D).
Clinical usefulness of the nomogram model
In the validation group and the entire cohort, the DCA of the nomogram model was presented in Figure 4. With a threshold probability of 0.13–0.55, using this model to identify elderly STEMI patients who might happen no-reflow after primary PCI would have a more significant net benefit than the “treat all” or “treat none” strategies in the validation group. The threshold probability of 0–0.55 was the most beneficial for predicting no-reflow with our nomogram an entire cohort.
Discussion
The rapid restoration of IRA blood flow and the rescue of ischemic myocardium is the key to the treatment of AMI. The phenomenon of no-reflow is related to the area and mortality of myocardial infarction and can supply valuable predictive information, especially in elderly patients. In this study, a prediction nomogram model of no-reflow was developed and confirmed relied on a small sample size of elderly STEMI patients treated with PCI. This model showed excellent performance and clinically usefulness. Several prediction models or risk score systems have been constructed and confirmed to predict the no-reflow phenomenon in patients treated with primary PCI for STEMI (15-17). However, the population of these studies concentrated on all STEMI patients or female patients. To the best of our knowledge, as of the writing of this article, this present research was the first to establish a quantitative nomogram to predict the risk of no-reflow in elderly STEMI patients.
In this current study, the nomogram incorporated five indexes, including preoperative TIMI blood flow, the diameter of the target lesion, collateral circulation, pulse pressure, and the number of leads for ST-segment elevation. The related study showed that pre-PCI have sufficient TIMI flow has a strong association with post-procedural TIMI 3 flow, myocardial blush grade 2–3, and smaller enzymatic infarct size (18). Patients with a low TIMI flow (≤1) in the IRA before PCI had a higher rate of no-reflow than those with sufficient TIMI flow (≥2) according to baseline angiography. Zhou et al. (19) indicated that low TIMI flow grade (≤1) before primary PCI was the independent predictor of the no-flow phenomenon (OR =1.100, 95% CI: 1.080–1.250, P<0.001). Like earlier research results, this study also found that preoperative TIMI blood flow was an independent risk factor of no-reflow phenomenon in elderly STEMI patients. And, in the current nomogram model, the score of preoperative TIMI blood flow ≤ grade 1 was 28, which was much higher than the score of preoperative TIMI blood flow > grade 1.
With the increase of target lesions, the thrombus and plaque burden increases correspondingly (20). The related pieces of research have found that thrombus and plaque burden were associated with the no-reflow phenomenon (21,22). Kirma et al. (23) pointed out that compared with patients with a target lesion length ≤13.5 mm, those with a target lesion length >13.5 mm were 5.4-fold more likely to occur the no-reflow phenomenon. However, our study introduced that the diameter of the target lesion ≥3.5 mm, was an independent risk factor of no-reflow and included in the nomogram model. In addition, thrombotic lesions have been proved to be the difficulty of interventional treatment of STEMI. During the treatment process, thrombus is prone to fall off, causing distal thromboembolism, forming no reflow or slow blood flow. Removing thrombus before stent implantation can reduce the incidence of chronic flow and no reflow, and further improve the efficacy of PCI in the treatment of STEMI. In the process of removal, all thrombus should be removed to avoid the residual thrombus falling off and causing embolism. Overall, from another view, this study revealed the relationship between the diameter of the target lesion and the no-reflow phenomenon, which deserves further detailed study in the future.
Coronary collateral circulation is a protective mechanism of the heart and is a vascular channel that supplies narrowed blood vessels or infarcted myocardium. Accurate establishment of collateral circulation is crucial for reducing the area of myocardial infarction, delaying left ventricular remodeling, and reducing major cardiovascular events in patients with AMI. AMI patients with adequate collateral circulation before PCI treatment can protect coronary microcirculation and significantly reduce the incidence of no-reflow (24). Related research showed that collateral circulation is one of the independent predictors of no-reflow (25). This study found that the risk of no-reflow increased with a decrease in collateral circulation. When the number of collateral circulations is 0, the risk of no-reflow is the highest.
Pulse pressure, confirmed by both cardiac contraction and peripheral vascular resistance, can reflect the fluctuation of blood pressure. The study has shown that pulse pressure, no systolic, or diastolic blood pressure is one of the most important predictors of coronary heart disease risk (26). In STEMI patients undergoing PCI, low pulse pressure can cause a reduction of coronary blood flow, resulting in inadequate myocardial perfusion. Decreased coronary blood flow can increase capillary blood vessels to capture leukocytes, and promote leukocyte adhesion and aggregation, thereby aggravating the occurrence of the no-reflow phenomenon. The predictive model constructed in this study showed that the decline of pulse pressure increased the risk of no-reflow after PCI in elderly STEMI patients.
At present, the effect of myocardial reperfusion on STEMI patients after IRA is preliminarily judged clinically by observing the depression of ST associated with the elevation of infarct-related lead in the ECG. The rapid fall of the ST segment marks the recovery of the coronary artery forward flow, and the structure and function of the microcirculation have not been damaged. In this study, it is found that with the increased number of leads for ST-segment elevation, its score in the nomogram model also showed an upward trend. It may be related to the failure of effective reperfusion in ischemic myocardium and insufficient rapid correction of extracellular potassium ion concentration, which leads to an insufficient decline in the ST-segment of ECG.
This study had the following shortcomings. Firstly, because this study is a retrospective study, some unknown factors is prone to data deviation or record deviation and lead to inevitable bias. The patients with incomplete clinical information were excluded and the enrolled cases were randomly grouped. Secondly, study found that serious complications such as heart failure after PCI can aggravate the patient’s condition and even lead to sudden death, however, the time interval of this study is relatively long and prognostic data is lacking, research and statistics of complications are easy to miss. Another shortcoming of this study is that the number of patients in this study is small, so that the constructed model has specific clinical significance, but not obvious. Also, our research lacked external validation for the nomogram, and large-scale studies are needed for verification. Besides, the case data in this study are all from the same hospital, which may lead to errors in results due to excessive consistency between the treatment method and the external environment. Other hospitals or databases should be added for external verification. In addition, this study only analyzed the risk factors of patients without reflow, and ignored the prediction of reflow after PCI. In the future work, to improve and optimize this prediction model, we will combine the previous research to screen further the independent risk factors of the occurrence of reflow/no-reflow phenomenon in elderly patients with STEMI after PCI treatment. It is believed that our findings will eventually become a more detailed and accurate prediction system for clinical work and provide a reference for the elaboration of personalized treatment plans for patients after PCI.
In conclusion, we constructed and confirmed a nomogram model, supplying an individual no-reflow prediction for elderly STEMI patients who received primary PCI treatment. This nomogram model would additionally play a clinical role, help to find the high-risk population after PCI, and improve the prognosis of elderly STEMI patients.
Acknowledgments
Funding: This work was supported by Tianjin Health Science and Technology Project (ZC20078).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-8003
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-8003
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-8003). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This work was conducted following the Declaration of Helsinki (2013) of the World Medical Association. The study was approved by ethics board of Tianjin Chest Hospital (No: 2020YS-040-01). Because the study was a retrospective cohort study, informed consent was abandoned.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mensah GA, Roth GA, Fuster V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J Am Coll Cardiol 2019;74:2529-32. [Crossref] [PubMed]
- Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol 2019;16:203-12. [Crossref] [PubMed]
- Reindl M, Reinstadler SJ, Feistritzer HJ, et al. Acute myocardial infarction as a manifestation of systemic vasculitis. Wien Klin Wochenschr 2016;128:841-3. [Crossref] [PubMed]
- Lattuca B, Kerneis M, Zeitouni M, et al. Elderly Patients with ST-Segment Elevation Myocardial Infarction: A Patient-Centered Approach. Drugs Aging 2019;36:531-9. [Crossref] [PubMed]
- Vogel B, Claessen BE, Arnold SV, et al. ST-segment elevation myocardial infarction. Nat Rev Dis Primers 2019;5:39. [Crossref] [PubMed]
- Ottani F, Limbruno U, Latini R, et al. Reperfusion in STEMI patients: still a role for cardioprotection? Minerva Cardioangiol 2018;66:452-63. [PubMed]
- Kalra S, Bhatt H, Kirtane AJ. Stenting in Primary Percutaneous Coronary Intervention for Acute ST-Segment Elevation Myocardial Infarction. Methodist Debakey Cardiovasc J 2018;14:14-22. [PubMed]
- Koutsoukis A, Kanakakis I. Challenges and unanswered questions in STEMI management. Hellenic J Cardiol 2019;60:211-5. [Crossref] [PubMed]
- Brosh D, Assali AR, Mager A, et al. Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality. Am J Cardiol 2007;99:442-5. [Crossref] [PubMed]
- Jaffe R, Dick A, Strauss BH. Prevention and treatment of microvascular obstruction-related myocardial injury and coronary no-reflow following percutaneous coronary intervention: a systematic approach. JACC Cardiovasc Interv 2010;3:695-704. [Crossref] [PubMed]
- Berg R, Buhari C. Treating and preventing no reflow in the cardiac catheterization laboratory. Curr Cardiol Rev 2012;8:209-14. [Crossref] [PubMed]
- Bouleti C, Mewton N, Germain S. The no-reflow phenomenon: State of the art. Arch Cardiovasc Dis 2015;108:661-74. [Crossref] [PubMed]
- Harrison RW, Aggarwal A, Ou FS, et al. Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol 2013;111:178-84. [Crossref] [PubMed]
- Ndrepepa G, Tiroch K, Keta D, et al. Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv 2010;3:27-33. [Crossref] [PubMed]
- Wang JW, Chen YD, Wang CH, et al. Development and validation of a clinical risk score predicting the no-reflow phenomenon in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Cardiology 2013;124:153-60. [Crossref] [PubMed]
- Wang JW, Zhou ZQ, Chen YD, et al. A risk score for no reflow in patients with ST-segment elevation myocardial infarction after primary percutaneous coronary intervention. Clin Cardiol 2015;38:208-15. [Crossref] [PubMed]
- Chen Y, Wang C, Yang X, et al. Independent no-reflow predictors in female patients with ST-elevation acute myocardial infarction treated with primary percutaneous coronary intervention. Heart Vessels 2012;27:243-9. [Crossref] [PubMed]
- De Luca G, Ernst N, Zijlstra F, et al. Preprocedural TIMI flow and mortality in patients with acute myocardial infarction treated by primary angioplasty. J Am Coll Cardiol 2004;43:1363-7. [Crossref] [PubMed]
- Zhou H, He XY, Zhuang SW, et al. Clinical and procedural predictors of no-reflow in patients with acute myocardial infarction after primary percutaneous coronary intervention. World J Emerg Med 2014;5:96-102. [Crossref] [PubMed]
- Bae JH, Kwon TG, Hyun DW, et al. Predictors of slow flow during primary percutaneous coronary intervention: an intravascular ultrasound-virtual histology study. Heart 2008;94:1559-64. [Crossref] [PubMed]
- Claeys MJ, Bosmans J, De Ceuninck M, et al. Effect of intracoronary adenosine infusion during coronary intervention on myocardial reperfusion injury in patients with acute myocardial infarction. Am J Cardiol 2004;94:9-13. [Crossref] [PubMed]
- Tanaka A, Kawarabayashi T, Nishibori Y, et al. No-reflow phenomenon and lesion morphology in patients with acute myocardial infarction. Circulation 2002;105:2148-52. [Crossref] [PubMed]
- Kirma C, Izgi A, Dundar C, et al. Clinical and procedural predictors of no-reflow phenomenon after primary percutaneous coronary interventions: experience at a single center. Circ J 2008;72:716-21. [Crossref] [PubMed]
- Desch S, Eitel I, Schmitt J, et al. Effect of coronary collaterals on microvascular obstruction as assessed by magnetic resonance imaging in patients with acute ST-elevation myocardial infarction treated by primary coronary intervention. Am J Cardiol 2009;104:1204-9. [Crossref] [PubMed]
- Albertal M, Cura F, Escudero AG, et al. Relationship between collateral circulation and successful myocardial reperfusion in acute myocardial infarction: a subanalysis of the PREMIAR trial. Angiology 2008;59:587-92. [Crossref] [PubMed]
- Glasser SP, Halberg DL, Sands C, et al. Is pulse pressure an independent risk factor for incident acute coronary heart disease events? The REGARDS study. Am J Hypertens 2014;27:555-63. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


