Biology of MET: a double life between normal tissue repair and tumor progression
MET structure and its activation
MNNG HOS transforming gene (MET) is a class IV receptor tyrosine kinase, a single pass transmembrane protein with an extracellular domain, a transmembrane hydrophobic sequence and an intracellular portion. The intracellular sequence has tyrosine kinase activity and is necessary for signal transduction (Figure 1).
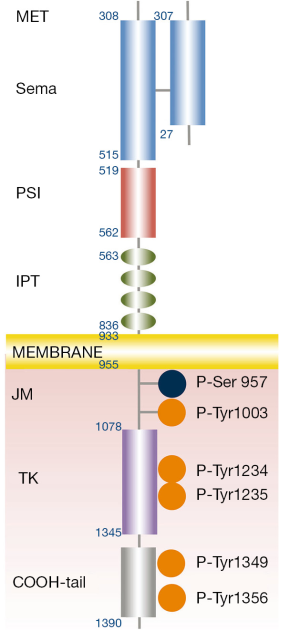
MET is translated as a single chain precursor of 1,390 amino acids (NM_000245). After translation, the protein is transported to the Golgi reticulum and is glycosylated (1). In the post-Golgi compartment, the cellular protease furin cleaves MET precursor between residues 307 and 308, in two chains: alpha (the N-terminal portion) and beta (2). The two chains remain linked by a disulphide-bound forming a heterodimer in the extracellular portion of the receptor (2). The extracellular portion is composed of three domains: a SEMA (semaphorin) domain, a PSI (plexin-semaphorin-integrin) domain and four IPT (immunoglobulin-plexin-transcription) repeats. A large SEMA domain, extended for the first 514 N-terminal residues, includes the whole alpha and part of the beta subunits (308-514 aa) (2). This sequence shares sequence homology with the domains of the semaphorin and plexin families. The structure of the SEMA domain is a seven-bladed beta-propeller and includes the binding site for the hepatocyte grow factor (HGF) (3). PSI domain follows the SEMA and counts about 50 residues with four disulphide bonds. Four IPT domains, which are related to immunoglobulin-like domains, connect the PSI domain with the transmembrane helix. The immunoglobulin-like domains are considered a stalk exposing the SEMA domain to the ligand. The intracellular portion includes the juxtamembrane sequence, the tyrosine kinase catalytic sites and the carboxyl-terminal sequences. The tyrosine kinase activity is increased by the phosphorylation of Tyr1234 and Tyr1235 within the catalytic site and is repressed by that of Ser975 within the juxtamembrane portion (2). Two other tyrosines: Tyr1349 and Tyr1356 belong to the carboxyl-terminal tail and act as a docking site for the recruitment of multiple transducers and adaptors (2).
Gene and transcripts
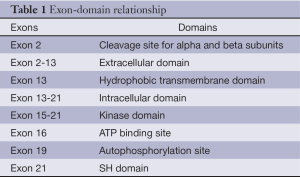
MET locus is mapped on chromosome 7q21-31 (chr7: 116312411-116438440 according to NCBI 37) and counts 21 exons. The entire first exon is not translated (5’-UTR) as well as the first 14 nucleotides of exon 2 after which the coding sequence begins. Exon 13 contains the sequence that encodes for the hydrophobic transmembrane domain. Therefore, exons 14-21 represent the intracellular domains (Table 1). At least three different transcript isoforms of MET have been described originating from alternative splicing (4). The most commonly expressed isoform in human tissue and cell lines (NM_000245.2) lacks of 54 nucleotides of exon 10 (5). Alternative splicing of exon 14 generates another isoform with an in-frame deletion of 47 amino acids in the juxtamembrane domain, which lacks of the Tyr1003 necessary for CBL binding and protein degradation (6). This isoform has been associated to pathological process such as cancer growth because of the reduced receptor internalization and degradation (7). MET promoter lacks TATA or CAAT elements and contains four putative binding sites for the transcription factors ETS, indeed ETS1 induces MET transcription in vitro (2,4). Moreover, MET promoter has hypoxia response elements (HREs) that can bind to HIF1 during hypoxic conditions (2).
Full table
Intracellular MET signaling
MET has been observed on the cell surface in monomer and dimers (8). HGF induces homo-dimerization and phosphorylation of two tyrosine residues (Tyr1234 and Tyr1235) of the catalytic loop of the kinase domain. Subsequently, the tyrosine residues Tyr1349 and Tyr1356 of the carboxy-terminal tail become phosphorylated forming a tandem SH2 recognition motif (9) that is able to recruit several signaling effectors including GRB2, SHC, CRK, PI3K, PLCγ, SRC, SHIP2 and STAT3 (10).
Gab1 knock out embryos show the same defects of those MET or HGF null (11,12). The GAB1 adaptor protein can bind directly to phosphorylated MET or through GAB2 and creates the binding site for more downstream adaptors (1). The direct interaction requires 13 amino acids of GAB1 that constitute the binding site for MET and interact directly with Tyr1349 of the carboxyl-terminal tail (13). This unique interaction sustains activation of several signaling pathways recruited by GAB1. The indirect interaction with GRB2 not only recruits GAB1 but also is necessary for KRAS activation leading to tumorigenesis and metastatic spread (14,15).
The complete plethora of downstream signaling of MET has not been fully elucidated; different pathways can be observed in different tissues (2). However, large-scale phospoproteomics studies demonstrated highly conserved core elements of MET signaling (16-18). In the presence of oncogene addiction, specific inhibitors determine dephosphorylation of elements belonging to similar intracellular pathway in EGFR mutant and MET amplified cells (18).
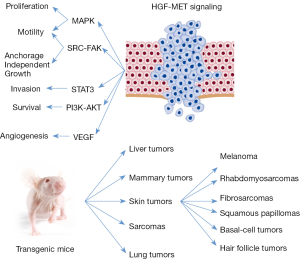
Indeed, the activation of MET has been linked to the best-known intracellular signaling pathways (Figure 2). Through the activation of MAPK pathway, MET can stimulate cell proliferation, cell cycle progression and cell mobility (19,20). The activation of KRAS and the downstream MAPK pathway occurs through the binding of SHC and GRB2 to the activated MET (21).
MET can promote cell survival through PI3K-AKT pathway. The p85 subunit of PI3K can bind the activated MET directly or indirectly through GAB1 (22). The activation PI3K-AKT pathway induces the transcription of the anti-apoptotic proteins BCL2 and BCL-XL that sustain the pro-survival signal (23). Together with SRC, PI3K-AKT is an intermediary for the MET dependent activation of NF-κB (24).
STAT3 can directly bind to MET and after phosphorylation can migrate to the nucleus to induce tubulogenesis (25) and invasion (26).
The activation of MET-SRC-FAK axis leads to cell migration and promotion of anchorage independent cell grow (27,28). Moreover, SRC activation induces a positive feedback on MET activation (16,27).
HGF exerts pro-angiogenic properties promoting the formation of blood vessels (29) through the induction of the vascular endothelial growth factor (VEGF) and the inhibition of thrombospondin, a negative regulator of angiogenesis (30,31).
Several phosphatases negatively regulate MET receptor including LAR that inhibits MET’s activation and induces contact inhibition of cell growth. Moreover, DEP1 (32), PTP1B, and PTP1B phosphatases regulate phosphorylation of MET (33). Negative regulation of MET signaling occurs by phosphorylation of tyrosine Tyr1003 in the juxtamembrane domain (2). The phosphorylation of tyrosine Tyr1003 in the juxtamembrane domain is necessary for CBL E3-ligase binding through SH2-like TKB domain of the CBL (34). CBL binding determines ligand-dependent ubiquitination of MET, internalization of the receptor and subsequently its lysosomal degradation (2). MET Tyr1003 mutants cannot bind to CBL and result in an enhanced stability of MET that is responsible for transformation and tumorigenic properties (2,35). MET degradation can occur also in an ubiquitin independent manner that does not require the kinase activity of the receptor. The disintegrine metalloprotease ADAM cleaves the extracellular NH terminal portion of MET (receptor shedding). Whereas membrane anchored cytoplasmic tail undergoes proteasome degradation, the extracellular domain is released and can sequester HGF or bind the full length MET preventing its activation (36). The treatment with monoclonal antibodies anti-MET increases the shedding of the receptor (36).
Beside the classical activation due to HGF, MET can interact with several cell surface proteins including semaphorin (37), beta-4 integrin (38), and CD44 (39). The interaction between MET and beta-4 integrin determines invasive growth (38), whereas that with CD44 links MET to actin and cytoskeleton (39). Because of the similarities in the extracellular SEMA-domain some semaphorines can activate MET in absence of HGF inducing MET dependent biological responses such as invasion (36). A putative MET-HER2 interaction induces loss of epithelial polarity and enhances invasion, in three-dimensional epithelial cell cultures (40).
Biological function of HGF-MET signaling
MET is present on the surface of epithelial cells of multiple organs including liver, pancreas, prostate, kidney, lung and bronchus (41,42). HGF, also known as scatter factor, is secreted by mesenchymal cells as a single chain precursor that becomes active after the cleavage by extracellular proteases into alpha and beta subunits. These subunits remain linked by a disulphide bound. In vitro, HGF is a potent inductor of proliferation for primary culture of hepatocytes and renal tubule cells and stimulates cell dissociation and sprouting (43).
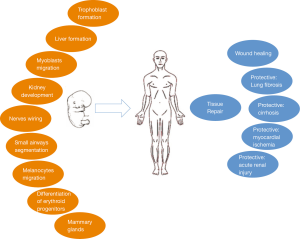
In vivo, HGF is a pleiotropic factor that stimulates proliferation, survival, motility, scattering and differentiation (Figure 3) (2). For example, HGF plays a direct role in proliferation and differentiation of erythroid progenitors (44). The paracrine loop between HGF secreted by mesenchymal cells and its receptor MET, present on epithelial and endothelial cells, promotes the migration and proliferation of stem cells that is necessary for tissue repair and wound healing (45-48).
HGF exerts a protective role in lung fibrosis (49) and liver cirrhosis (50). The activation of HGF-MET signaling in cells of kidney’s tubules exerts a protective effect after acute injury (51). Similarly, the activation of MET protects myocardiocytes during revascularization after ischemia (48). During tissue repair, several cytokines, present in the interstitial compartment including IL-1, IL-6, TNF-α and TGF-β, induce transcription of HGF and of MET in fibroblast-macrophages and in epithelial cells, respectively. Therefore, HGF becomes abundant and activated in the interstitial compartment during inflammation. This leads to MET activation as part of the physiological defense to tissue damage (36).
HGF induces cell scattering: a phenomena characterized by the lost of cell-cell contacts mediated by cadherin: allowing the cells to migrate (52). Madine–Darby cell line responds to HGF stimulation by scattering in two-dimensional cultures and forming tubules in three-dimensional cultures. These are key epithelial functions in wound repair and embryogenesis (53).
MET is necessary for the normal development of muscles because affects the migration of muscular cell precursors and for formation of the liver and the placenta since provides proliferation and survival signals for hepatocytes and trophoblastic cells (54,55). In c-Met homozygous mutant (−/−) mouse embryos, the skeletal muscles of the limb and diaphragm do not form because of the myogenic precursor cells do not colonize the limb bud and diaphragm. In contrast, the axial skeletal muscles present a normal development in the absence of c-Met signaling (55). Mice lacking HGF fail to complete development and die in utero. The mutation affects the embryonic liver, which is reduced in size and shows extensive loss of parenchymal cells. In addition, development of the placenta, particularly of trophoblast cells, is impaired resulting in a hypomorphic organ that causes in utero lethality (56,57). Transgenic mice lacking EGFR and MET signaling present smaller kidneys and a reduced number of nephrons (58).
On the contrary, transgenic mice with HGF under the control of metallothionein promoter present an increased size of their liver: about a double ratio of liver/body weight (59). A dramatic increase of 2N small hepatocytes is observed in transgenic livers. Hepatocytes isolated by perfusion of transgenic livers show a doubling time of 2 days in culture compared to no growth of wild type ones (59). This proliferation is sustained by chronic activation of MET and its downstream pathways. Indeed, transgenic mice have a much faster liver regeneration than controls after partial hepatectomy (59). Transgenic mice expressing HGF under the albumin promoter present lower level of HGF compared to those with HGF under the metallothionein promoter and a milder increase of liver size (60). Moreover, transgenic mice with HGF under the control of metallothionein promoter exhibit ectopic skeletal muscles and melanocytes in the central nervous system (61). Finally, the disruption of HGF-MET signaling alters the formation of nervous system connections with a reduced survival of sensory and sympathetic neurons and reduced outgrowth of some motor nerves (62-64).
MET in human cancers
HGF-MET signal promotes detachment of normal cells, without the activation of anoikis allowing their migration. This is necessary for the formation of several organs during embryogenesis and in adult life for tissue repair and wound healing. Cancer cells resume physiological programs, normally activated during embryogenesis, to achieve and enhance invasiveness and metastatic spread.
An altered form of MET (TPR-MET), of 65KDa, with constitutive kinase activity, initially named MNNG-HOS, has been cloned as a transforming factor from a chemically induced human osteosarcoma cell line and therefore, MET is considered a proto-oncogene (65). The cloned transcript is able to transform NIH-3T3 cells in vitro. The fusion protein originated from the translocation t(1q25;7q31) juxtaposes TPR (translocated promoter region) and the intracellular domain of MET (66,67). The fusion protein forms dimers through the leucine zipper encoded by the TPR portion resulting in a constitutively activated MET even in the absence of its ligand (68).
MET overexpression is observed in many human tumors (www.vai.org/HgfSf-MET and cancer), which is, frequently, associated with a metastatic phenotype and poor prognosis (43). In colorectal cancer, MET amplification and expression correlates with a more advanced stage, tumor invasiveness and presence of metastases both in lymph node and liver (69,70). Similar results have been reported in ovarian (71-73) and breast cancers (74). In non-small cell lung cancer (NSCLC), MET is overexpressed in 25-75% of the cases and is associated with poor prognosis (75-81). The contextual overexpression of HGF can generate a paracrine loop that possibly sustains the cancer growth such as demonstrated in transgenic animals (82). Amplification of MET has been described in a minority of NSCLCs (1.4-7%) (83-85) and in gastric (86), esophageal (87), colorectal (88) and clear cell ovarian cancer (89).
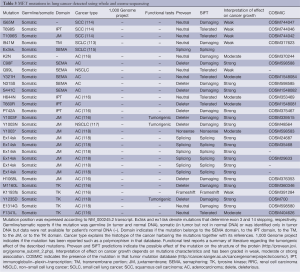
Germline MET mutations have been observed in patients with hereditary papillary renal cell carcinomas (HPRCC) providing the first secure evidence of a link between MET and human oncogenesis (90). Papillary renal cell carcinoma accounts for 10-15% of primary kidney tumors. A familial syndrome, associated with germline mutations of MET, is responsible for a minority of the cases and induces tumors with an incomplete penetrance. These mutations interest the tyrosine kinase domain of MET (90), are able to induce auto phosphorylation of the receptor and to transform NIH 3T3 cells in vitro (Table 2) (90). Mutant and wild type MET have been transfected in NIH-3T3 cells. The mutant isoforms more potently induce tumor formation after injection in nude mice (110). Only 13% of the sporadic papillary renal cell carcinomas present MET mutations (103). In these tumors, germline and somatic MET mutations have been described (103). Somatic MET mutations are more tumorigenic because induce more transformation of NIH-3T3 cells in vitro and xenograft proliferation in vivo (110). This suggests a negative selection of the more potent activating mutations during embryogenesis and development (110). Moreover, the germline mutations, observed in sporadic papillary renal cell carcinomas, show tumorigenic activity. This implies a de novo mutation; a different occurrence than single nucleotide polymorphisms not related to cancer.
Moreover, somatic mutations of MET tyrosine kinase domain have been described in head and neck squamous cell carcinomas (Tyr1248C and Tyr1253) (71), in a glioma (Gly1137Val) (107) and in a mucinous ovarian carcinoma (Ala1209Gly) (109). In childhood hepatocellular carcinomas, mutations affected both the tyrosine kinase domain (Thr1191Ile) and the carboxyl-terminal tail (Lys1262Arg and Met1268Ile) (108).
More difficult is the interpretation of mutations reported exclusively in tumor DNA without knowing the status of patients’ normal DNA. This is the case of several reports regarding NSCLCs, small cell lung cancers (SCLCs) and mesotheliomas (Table 2). In these tumors mutations in the SEMA and juxtamembrane domains have been reported (7,91,96,97,99,100). It is difficult to understand their impact on cancer growth in the absence of a wild type allele in normal DNA or outside the context of a familiar cancer related syndrome. Recently, data from the 1,000 genome project have become available showing that some of the putative MET mutations are also present in normal subjects and possibly polymorphisms. These mutations include Thr1010Ile, Arg988Cys and Asn375Ser (111). Different consideration should be made for the somatic mutation observed in NSCLC that determines the skipping of exon 14 (97). Exon 14 contains the tyrosine Tyr1003 which phosphorylation allows CBL binding with subsequent internalization and disruption of the receptor (112).

Full table
Recently, high throughput molecular evaluations of lung cancers have demonstrated somatic missense mutations in SCLC (113) and squamous cell carcinomas (Table 3) (114). Interestingly, in adenocarcinomas of the lung, MET locus was included in a peak of amplification according to GISTIC analysis strongly indicating its relevance for the growth of these tumors (118). Moreover, MET was significantly mutated according to MutSig2CV algorithm (Table 3). RNA sequencing demonstrated MET exon 14 skipping in 4% of cases (118). Data indicate the relevance of MET genomic aberrations for the growth of lung adenocarcinomas and suggest that MET could represent a target for therapy. Finally, MET amplification has been described such as a cause of acquired resistance during treatment with tyrosine kinase inhibitors in patients with EGFR mutations (83,119).
Full table
In vivo models of HGF-MET driven tumors
Coexpression of wild type MET and HGF in the same NIH 3T3 cell generates an autocrine loop that increases tumor formation and metastatic dissemination when cells are implanted in nude mice (120). On contrary, uncleavable form of HGF binds with high affinity MET and inhibits its activation (121). The local expression of uncleavable HGF within xenograft suppresses tumor growth, impairs tumor angiogenesis, and prevents metastatic dissemination; whereas the systemic expression of uncleavable HGF dramatically inhibits the growth of transplanted tumors and abolishes the formation of spontaneous metastases (121).
HGF-MET pathway is relevant for the growth of lung tumors. When recombinant HGF was injected within SCID mice’s xenograft of lung adenocarcinoma cells, tumor showed a 3-fold larger volume than saline-injected controls (122).
Transgenic mice with HGF under control of metallothionein promoter show an increased rate formation of hepatocellular carcinomas and adenomas. Tumors are developed with a long latency because observed only after 17 months of age in FVB/N strain (59). Tumors originate from both epithelial and mesenchymal cells in a wide variety of tissues. Most frequently, tumors arise in the female mammary gland but also skin tumors are common, including melanoma, rhabdomyosarcomas, fibrosarcomas, squamous papillomas, basal-cell and hair follicle tumors. Met phosphorylation is observed in these tumors, suggesting that autocrine signaling broadly promotes tumorigenesis (123). In the same model, HGF overexpression promotes hepatocarcinogenesis and VEGF induces angiogenesis after treatment with diethylnitrosamine, a well-characterized genetic mutagen (124). However, contradictory results have been reported, Shiota et al. describe that the overexpression of the human full-length HGF isoform under the transcriptional regulation of the albumin promoter in mice (FVB genetic background) do not induce hepatocellular carcinoma development (60). Moreover, the cross breading of Hgf and Myc transgenic mice determines a dramatic inhibition of hepatocarcinogenesis in the hybrids (125).
The transgenic mice, overexpressing HGF under metallothionein promoter, present an inappropriate abundance of melanocytes in the dermis, dermo-epithelial junction and in basal layer of the skin (61); an increased number of melanomas are present in these animals (126). However, there is a long latency before these mice develop melanomas. The treatment with carcinogenetic agents (7,12-dimethylbenz[a]anthracene and 12-O-tetradecanoylphorbol-13-acetate) strongly increases the number and the formation of metastatic melanomas in transgenic mice that overexpress HGF (127). The gain of function mutation of the cell cycle control cyclin (CDK4) and the deletion of its inhibitor CDKN2A (p19INK) are key genetic events frequently observed in melanomas (128,129). Transgenic mice with HGF overexpression that bear CDK4Arg24Cys mutation present a dramatic increase in the number of carcinogen-induced primary melanomas of the skin and their metastasis (127).
Similar results are observed in mice overexpressing met and its oncogenic isoforms. Transgenic mice overexpressing met under the control of metallothionein 1 promoter present severe breading defects. Two mammary adenocarcinomas have been observed in met overexpressing mice with Met1268Thr and Tyr1248His mutations (130). When the expression of met is limited to hepatocytes and posed under the control of doxycycline, transgenic mice develop in sequence hyperplastic foci, dysplastic liver foci, and overt tumors either hepatocellular carcinomas or hepatocellular adenomas by 3 months of age (131). Hepatocellular carcinomas do not develop within hepatocellular adenomas and vice versa. Whereas all the hepatocytes expressed transgenic MET, only those of neoplastic and dysplastic foci expressed an activated receptor (phosphorylation of Tyr1234 and Tyr1235) (131). The development of hepatocellular carcinomas or hepatocellular adenomas depends on the occurrence of additional genetic events: activation of beta-catenin or inactivation of the HNF1α pathway, respectively (131). The inactivation of the transgenic met by administration of doxycycline leads to regression of hepatocellular carcinomas in the animals (132) supporting the idea that MET could be an effective target for therapy despite the presence of additional mutations in the same tumor.
Truncation of MET extracellular domain generates a constitutively activated tyrosine kinase: the intracellular portion of MET (cytoMET) is a weak transforming factor (133). Transgenic mice expressing cytoMET in the liver under the control of α1-antitripsine have been generated. These mice do not develop liver tumors during their life and are more resistant to pro-apoptotic stimuli (activation of FAS receptor through JO2 antibody). Hepatocyte cell lines can be established from their liver and maintain a differentiate phenotype and do not undergo transformation (134). Human MET is not activated by murine HGF (135,136). Four lines of transgenic mice have been created in order to express human MET in murine hepatocytes in absence of doxycycline (132). Mice of lines 1 and 2 were born with fatty liver and died within 2 months from the partum. If feed with doxycycline during the pregnancy and for 4 weeks after partum, mice grew normally until 10 months when started to die; 85% of these mice developed hepatocellular carcinomas. Mice of lines 3 and 4 were healthy at birth but started to die after 4 months: about 85% of them for hepatocellular carcinomas (132). Mice firstly developed foci of hyperplasia that then progressed to malignancy (hepatocellular carcinomas). Treating mice bearding hepatocellular carcinomas with doxycycline, to inhibit MET expression, determined the regression of the nodules (132). Authors showed that the overexpression of MET in presence of cell adherence was sufficient to activate MET signaling since none of the oncogenic mutations of MET were present and murine HGF was ineffective to activate MET signaling (132).
Transgenic mice expressing human oncogenic protein TRP-MET under the control of metallothionein 1 promoter mainly develop mammary gland tumors (137).
Met mutations have been knocked-in in four C57BL/6 mouse lines harboring: Asp1226Asn, Tyr1228Cys, Met1248Thr and the double mutation Met1248Thr/Lys1193Val (138). The mutated forms of MET were expressed under the control of the endogenous Met promoter. Despite the strong activity of these mutations in vitro, an unexpected long latency before tumor formation is observed (138). Different mutations induce different type of tumors: MET1248Thr(mu/+) develops only carcinomas, whereas Asp1226Asn(mu/mu), Tyr1228Cys(mu/+), and MET1248Thr/Lys1193Val(mu/+) develop also sarcomas and lymphomas (138). In the tumor cells of mice with Met mutations, amplifications of the mutated allele are observed, regardless of the type of mutation (138,139). When knock-in activating alleles are express in the FVB background, tumor latency significantly decrease and a higher penetrance is observed (140). Breast adenocarcinomas are common in all FVB lines, whereas are not observed in C57BL/6 mice. Mice bearing METAsp1226Asn mutation frequently develop hemangiosarcomas, whereas those with METMet1248Thr rarely develop sarcomas (140).
The mouse mammary tumor virus (MMTV) promoter allows the expression of MET in the mammary epithelium. The overexpression of MET and its oncogenic isoforms (Met1248Thr, Tyr1003Phe/Met1248Thr) induces tumor formation with low penetrance and a long latency. Mice develop breast carcinomas: 50% with solid nodular histology and 50% with papillary, shirrous, adeno-squamous or spindle cell phenotype (141).
The SWR/J strain of mice is predisposed to develop lung adenomas, spontaneously. This phenomena is under polygenic control and susceptibility alleles have been identified including pulmonary adenoma-susceptibility 1 (Pas1), -resistance 2 and 4 (Par2 and Par4) loci (142). The difference between SWR/J and BALBc mice, respectively susceptible and resistant to develop lung adenomas, depends on the Par4 allele, a narrow region of the murine chromosome 6. Sequencing of this region revealed a single amino-acid change, consisting in a non-conservative Arg968Cys variation in the juxtamembrane domain of met. BALB/c mice carry the Arginine allele, whereas the SWR/J mice strain the Cys variation (142). The corresponding Arg988Cys mutation in humans is observed as germline in one lung adenocarcinoma out of 126 (142) and in 2 SCLC cell lines (92). Arg988Cys mutation increases tumorigenicity in in vitro models (92).
Transgenic mice have been created in order to express human HGF exclusively in small airway and therefore the rat Clara cell secretory protein promoter was used (143). These mice develop small airways alterations with congestion, wide bifurcations and increased blood vessels formation. HGF transgenic mice are more susceptible to nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco carcinogen that induces lung tumors. Mice treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, present an increased number of tumors if overexpressing HGF (143). Mice develop papillary adenomas by 10-20 weeks and papillary or solid adenocarcinomas by 30-38 weeks. Tumors arose in this animal model have characteristics of both Clara cells and Type II pneumocytes.
Conclusions
HGF-MET signaling is necessary during embryogenesis for myoblast migration and liver and placenta formation. In adult life, HGF-MET signaling guides tissue repair and exerts protective effects during injury of multiple organs. In tumors, HGF-MET signaling leads to invasion, angiogenesis, metastatic spread, proliferation and anti-apoptosis. The paracrine activation of HGF-MET signal forms a loop, sufficient to sustain tumorigenesis in experimental models. However, genomic aberrations of MET have been described in human tumors; for example amplification and mutations have been reported in in lung adenocarcinomas. Experimental evidences demonstrate that the overexpression of MET and some of its mutants enhance cancer growth. Matter of debate remains which target will be useful for a specific therapy: just the autocrine loop, the protein overexpression, gene amplification or the presence of mutations.
This is relevant for the design of effective clinical trials with anti-MET agents.
Acknowledgements
University of Pisa funded this review.
Disclosure: The author declares no conflict of interest.
References
- Wu YM, Liu CH, Huang MJ, et al. C1GALT1 enhances proliferation of hepatocellular carcinoma cells via modulating MET glycosylation and dimerization. Cancer Res 2013;73:5580-90. [PubMed]
- Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol 2011;3:S7-S19. [PubMed]
- Stamos J, Lazarus RA, Yao X, et al. Crystal structure of the HGF beta-chain in complex with the Sema domain of the Met receptor. EMBO J 2004;23:2325-35. [PubMed]
- Liu Y. The human hepatocyte growth factor receptor gene: complete structural organization and promoter characterization. Gene 1998;215:159-69. [PubMed]
- Rodrigues GA, Naujokas MA, Park M. Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol Cell Biol 1991;11:2962-70. [PubMed]
- Lee CC, Yamada KM. Identification of a novel type of alternative splicing of a tyrosine kinase receptor. Juxtamembrane deletion of the c-met protein kinase C serine phosphorylation regulatory site. J Biol Chem 1994;269:19457-61. [PubMed]
- Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res 2005;65:1479-88. [PubMed]
- Dietz MS, Hasse D, Ferraris DM, et al. Single-molecule photobleaching reveals increased MET receptor dimerization upon ligand binding in intact cells. BMC Biophys 2013;6:6. [PubMed]
- Ponzetto C, Bardelli A, Zhen Z, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 1994;77:261-71. [PubMed]
- Weidner KM, Di Cesare S, Sachs M, et al. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 1996;384:173-6. [PubMed]
- Itoh M, Yoshida Y, Nishida K, et al. Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol Cell Biol 2000;20:3695-704. [PubMed]
- Sachs M, Brohmann H, Zechner D, et al. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J Cell Biol 2000;150:1375-84. [PubMed]
- Schaeper U, Gehring NH, Fuchs KP, et al. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol 2000;149:1419-32. [PubMed]
- Fixman ED, Naujokas MA, Rodrigues GA, et al. Efficient cell transformation by the Tpr-Met oncoprotein is dependent upon tyrosine 489 in the carboxy-terminus. Oncogene 1995;10:237-49. [PubMed]
- Saucier C, Papavasiliou V, Palazzo A, et al. Use of signal specific receptor tyrosine kinase oncoproteins reveals that pathways downstream from Grb2 or Shc are sufficient for cell transformation and metastasis. Oncogene 2002;21:1800-11. [PubMed]
- Organ SL, Tong J, Taylor P, et al. Quantitative phospho-proteomic profiling of hepatocyte growth factor (HGF)-MET signaling in colorectal cancer. J Proteome Res 2011;10:3200-11. [PubMed]
- Hammond DE, Hyde R, Kratchmarova I, et al. Quantitative analysis of HGF and EGF-dependent phosphotyrosine signaling networks. J Proteome Res 2010;9:2734-42. [PubMed]
- Guo A, Villen J, Kornhauser J, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci U S A 2008;105:692-7. [PubMed]
- Paumelle R, Tulasne D, Kherrouche Z, et al. Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway. Oncogene 2002;21:2309-19. [PubMed]
- Fixman ED, Fournier TM, Kamikura DM, et al. Pathways downstream of Shc and Grb2 are required for cell transformation by the tpr-Met oncoprotein. J Biol Chem 1996;271:13116-22. [PubMed]
- Graziani A, Gramaglia D. Hepatocyte growth factor/scatter factor stimulates the Ras-guanine nucleotide exchanger. J Biol Chem 1993;268:9165-8. [PubMed]
- Xiao GH, Jeffers M, Bellacosa A, et al. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A 2001;98:247-52. [PubMed]
- Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol 2004;287:F7-16. [PubMed]
- Fan S, Gao M, Meng Q, et al. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene 2005;24:1749-66. [PubMed]
- Boccaccio C, Ando M, Tamagnone L, et al. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 1998;391:285-8. [PubMed]
- Syed ZA, Yin W, Hughes K, et al. HGF/c-met/Stat3 signaling during skin tumor cell invasion: indications for a positive feedback loop. BMC Cancer 2011;11:180. [PubMed]
- Hui AY, Meens JA, Schick C, et al. Src and FAK mediate cell-matrix adhesion-dependent activation of Met during transformation of breast epithelial cells. J Cell Biochem 2009;107:1168-81. [PubMed]
- Rahimi N, Hung W, Tremblay E, et al. c-Src kinase activity is required for hepatocyte growth factor-induced motility and anchorage-independent growth of mammary carcinoma cells. J Biol Chem 1998;273:33714-21. [PubMed]
- Grant DS, Kleinman HK, Goldberg ID, et al. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A 1993;90:1937-41. [PubMed]
- Saucier C, Khoury H, Lai KM, et al. The Shc adaptor protein is critical for VEGF induction by Met/HGF and ErbB2 receptors and for early onset of tumor angiogenesis. Proc Natl Acad Sci U S A 2004;101:2345-50. [PubMed]
- Zhang YW, Su Y, Volpert OV, et al. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci U S A 2003;100:12718-23. [PubMed]
- Palka HL, Park M, Tonks NK. Hepatocyte growth factor receptor tyrosine kinase met is a substrate of the receptor protein-tyrosine phosphatase DEP-1. J Biol Chem 2003;278:5728-35. [PubMed]
- Sangwan V, Paliouras GN, Cheng A, et al. Protein-tyrosine phosphatase 1B deficiency protects against Fas-induced hepatic failure. J Biol Chem 2006;281:221-8. [PubMed]
- Peschard P, Park M. Escape from Cbl-mediated downregulation: a recurrent theme for oncogenic deregulation of receptor tyrosine kinases. Cancer Cell 2003;3:519-23. [PubMed]
- Abella JV, Peschard P, Naujokas MA, et al. Met/Hepatocyte growth factor receptor ubiquitination suppresses transformation and is required for Hrs phosphorylation. Mol Cell Biol 2005;25:9632-45. [PubMed]
- Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 2010;11:834-48. [PubMed]
- Giordano S, Corso S, Conrotto P, et al. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol 2002;4:720-4. [PubMed]
- Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell 2001;107:643-54. [PubMed]
- Orian-Rousseau V, Chen L, Sleeman JP, et al. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev 2002;16:3074-86. [PubMed]
- Khoury H, Naujokas MA, Zuo D, et al. HGF converts ErbB2/Neu epithelial morphogenesis to cell invasion. Mol Biol Cell 2005;16:550-61. [PubMed]
- Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008;7:504-16. [PubMed]
- Tsao MS, Liu N, Chen JR, et al. Differential expression of Met/hepatocyte growth factor receptor in subtypes of non-small cell lung cancers. Lung cancer 1998;20:1-16. [PubMed]
- Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003;4:915-25. [PubMed]
- Galimi F, Bagnara GP, Bonsi L, et al. Hepatocyte growth factor induces proliferation and differentiation of multipotent and erythroid hemopoietic progenitors. J Cell Biol 1994;127:1743-54. [PubMed]
- Forte G, Minieri M, Cossa P, et al. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells 2006;24:23-33. [PubMed]
- Neuss S, Becher E, Woltje M, et al. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells 2004;22:405-14. [PubMed]
- Matsumoto K, Nakamura T. Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int 2001;59:2023-38. [PubMed]
- Nakamura T, Mizuno S, Matsumoto K, et al. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J Clin Invest 2000;106:1511-9. [PubMed]
- Watanabe M, Ebina M, Orson FM, et al. Hepatocyte growth factor gene transfer to alveolar septa for effective suppression of lung fibrosis. Mol Ther 2005;12:58-67. [PubMed]
- Ueki T, Kaneda Y, Tsutsui H, et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med 1999;5:226-30. [PubMed]
- Zhou D, Tan RJ, Lin L, et al. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int 2013;84:509-20. [PubMed]
- Zhu H, Naujokas MA, Park M. Receptor chimeras indicate that the met tyrosine kinase mediates the motility and morphogenic responses of hepatocyte growth/scatter factor. Cell Growth Differ 1994;5:359-66. [PubMed]
- Corso S, Comoglio PM, Giordano S. Cancer therapy: can the challenge be MET? Trends Mol Med 2005;11:284-92. [PubMed]
- Maina F, Casagranda F, Audero E, et al. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell 1996;87:531-42. [PubMed]
- Bladt F, Riethmacher D, Isenmann S, et al. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 1995;376:768-71. [PubMed]
- Schmidt C, Bladt F, Goedecke S, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 1995;373:699-702. [PubMed]
- Uehara Y, Minowa O, Mori C, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995;373:702-5. [PubMed]
- Ishibe S, Karihaloo A, Ma H, et al. Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development 2009;136:337-45. [PubMed]
- Sakata H, Takayama H, Sharp R, et al. Hepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth Differ 1996;7:1513-23. [PubMed]
- Shiota G, Wang TC, Nakamura T, et al. Hepatocyte growth factor in transgenic mice: effects on hepatocyte growth, liver regeneration and gene expression. Hepatology 1994;19:962-72. [PubMed]
- Takayama H, La Rochelle WJ, Anver M, et al. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci U S A 1996;93:5866-71. [PubMed]
- Helmbacher F, Dessaud E, Arber S, et al. Met signaling is required for recruitment of motor neurons to PEA3-positive motor pools. Neuron 2003;39:767-77. [PubMed]
- Maina F, Hilton MC, Andres R, et al. Multiple roles for hepatocyte growth factor in sympathetic neuron development. Neuron 1998;20:835-46. [PubMed]
- Maina F, Hilton MC, Ponzetto C, et al. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev 1997;11:3341-50. [PubMed]
- Cooper CS, Park M, Blair DG, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 1984;311:29-33. [PubMed]
- Park M, Dean M, Cooper CS, et al. Mechanism of met oncogene activation. Cell 1986;45:895-904. [PubMed]
- Dean M, Park M, Vande Woude GF. Characterization of the rearranged tpr-met oncogene breakpoint. Mol Cell Biol 1987;7:921-4. [PubMed]
- Rodrigues GA, Park M. Dimerization mediated through a leucine zipper activates the oncogenic potential of the met receptor tyrosine kinase. Mol Cell Biol 1993;13:6711-22. [PubMed]
- Takeuchi H, Bilchik A, Saha S, et al. c-MET expression level in primary colon cancer: a predictor of tumor invasion and lymph node metastases. Clin Cancer Res 2003;9:1480-8. [PubMed]
- Zeng ZS, Weiser MR, Kuntz E, et al. c-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett 2008;265:258-69. [PubMed]
- Di Renzo MF, Olivero M, Martone T, et al. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene 2000;19:1547-55. [PubMed]
- Wong AS, Pelech SL, Woo MM, et al. Coexpression of hepatocyte growth factor-Met: an early step in ovarian carcinogenesis? Oncogene 2001;20:1318-28. [PubMed]
- Sawada K, Radjabi AR, Shinomiya N, et al. c-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res 2007;67:1670-9. [PubMed]
- Garcia S, Dales JP, Jacquemier J, et al. c-Met overexpression in inflammatory breast carcinomas: automated quantification on tissue microarrays. Br J Cancer 2007;96:329-35. [PubMed]
- Benedettini E, Sholl LM, Peyton M, et al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am J Pathol 2010;177:415-23. [PubMed]
- Ichimura E, Maeshima A, Nakajima T, et al. Expression of c-met/HGF receptor in human non-small cell lung carcinomas in vitro and in vivo and its prognostic significance. Jpn J Cancer Res 1996;87:1063-9. [PubMed]
- Liu C, Tsao MS. In vitro and in vivo expressions of transforming growth factor-alpha and tyrosine kinase receptors in human non-small-cell lung carcinomas. Am J Pathol 1993;142:1155-62. [PubMed]
- Nakamura Y, Niki T, Goto A, et al. c-Met activation in lung adenocarcinoma tissues: an immunohistochemical analysis. Cancer Sci 2007;98:1006-13. [PubMed]
- Olivero M, Rizzo M, Madeddu R, et al. Overexpression and activation of hepatocyte growth factor/scatter factor in human non-small-cell lung carcinomas. Br J Cancer 1996;74:1862-8. [PubMed]
- Siegfried JM, Weissfeld LA, Luketich JD, et al. The clinical significance of hepatocyte growth factor for non-small cell lung cancer. Ann Thorac Surg 1998;66:1915-8. [PubMed]
- Xu L, Nilsson MB, Saintigny P, et al. Epidermal growth factor receptor regulates MET levels and invasiveness through hypoxia-inducible factor-1alpha in non-small cell lung cancer cells. Oncogene 2010;29:2616-27. [PubMed]
- Kuperwasser C, Chavarria T, Wu M, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A 2004;101:4966-71. [PubMed]
- Cappuzzo F, Janne PA, Skokan M, et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol 2009;20:298-304. [PubMed]
- Okuda K, Sasaki H, Yukiue H, et al. Met gene copy number predicts the prognosis for completely resected non-small cell lung cancer. Cancer Sci 2008;99:2280-5. [PubMed]
- Onozato R, Kosaka T, Kuwano H, et al. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol 2009;4:5-11. [PubMed]
- Nakajima M, Sawada H, Yamada Y, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer 1999;85:1894-902. [PubMed]
- Miller CT, Lin L, Casper AM, et al. Genomic amplification of MET with boundaries within fragile site FRA7G and upregulation of MET pathways in esophageal adenocarcinoma. Oncogene 2006;25:409-18. [PubMed]
- Umeki K, Shiota G, Kawasaki H. Clinical significance of c-met oncogene alterations in human colorectal cancer. Oncology 1999;56:314-21. [PubMed]
- Yamamoto S, Tsuda H, Miyai K, et al. Gene amplification and protein overexpression of MET are common events in ovarian clear-cell adenocarcinoma: their roles in tumor progression and prognostication of the patient. Mod Pathol 2011;24:1146-55. [PubMed]
- Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet 1997;16:68-73. [PubMed]
- Ma PC, Tretiakova MS, MacKinnon AC, et al. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes Cancer 2008;47:1025-37. [PubMed]
- Ma PC, Kijima T, Maulik G, et al. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res 2003;63:6272-81. [PubMed]
- Seiwert TY, Jagadeeswaran R, Faoro L, et al. The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res 2009;69:3021-31. [PubMed]
- Krishnaswamy S, Kanteti R, Duke-Cohan JS, et al. Ethnic differences and functional analysis of MET mutations in lung cancer. Clin Cancer Res 2009;15:5714-23. [PubMed]
- Tengs T, Lee JC, Paez JG, et al. A transforming MET mutation discovered in non-small cell lung cancer using microarray-based resequencing. Cancer Lett 2006;239:227-33. [PubMed]
- Jagadeeswaran R, Ma PC, Seiwert TY, et al. Functional analysis of c-Met/hepatocyte growth factor pathway in malignant pleural mesothelioma. Cancer Res 2006;66:352-61. [PubMed]
- Kong-Beltran M, Seshagiri S, Zha J, et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res 2006;66:283-9. [PubMed]
- Puri N, Ahmed S, Janamanchi V, et al. c-Met is a potentially new therapeutic target for treatment of human melanoma. Clin Cancer Res 2007;13:2246-53. [PubMed]
- Voortman J, Harada T, Chang RP, et al. Detection and therapeutic implications of c-Met mutations in small cell lung cancer and neuroendocrine tumors. Curr Pharm Des 2013;19:833-40. [PubMed]
- Ludovini V, Bianconi F, Pistola L, et al. Optimization of patient selection for EGFR-TKIs in advanced non-small cell lung cancer by combined analysis of KRAS, PIK3CA, MET, and non-sensitizing EGFR mutations. Cancer Chemother Pharmacol 2012;69:1289-99. [PubMed]
- Tyner JW, Fletcher LB, Wang EQ, et al. MET receptor sequence variants R970C and T992I lack transforming capacity. Cancer Res 2010;70:6233-7. [PubMed]
- Lee JH, Han SU, Cho H, et al. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene 2000;19:4947-53. [PubMed]
- Schmidt L, Junker K, Nakaigawa N, et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene 1999;18:2343-50. [PubMed]
- Olivero M, Valente G, Bardelli A, et al. Novel mutation in the ATP-binding site of the MET oncogene tyrosine kinase in a HPRCC family. Int J Cancer 1999;82:640-3. [PubMed]
- Salvi A, Marchina E, Benetti A, et al. Germline and somatic c-met mutations in multifocal/bilateral and sporadic papillary renal carcinomas of selected patients. Int J Oncol 2008;33:271-6. [PubMed]
- Lorenzato A, Olivero M, Patane S, et al. Novel somatic mutations of the MET oncogene in human carcinoma metastases activating cell motility and invasion. Cancer Res 2002;62:7025-30. [PubMed]
- Moon YW, Weil RJ, Pack SD, et al. Missense mutation of the MET gene detected in human glioma. Mod Pathol 2000;13:973-7. [PubMed]
- Park WS, Dong SM, Kim SY, et al. Somatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomas. Cancer Res 1999;59:307-10. [PubMed]
- Tanyi J, Tory K, Rigo J, et al. Evaluation of the tyrosine kinase domain of the Met proto-oncogene in sporadic ovarian carcinomas*. Pathol Oncol Res 1999;5:187-91. [PubMed]
- Jeffers M, Schmidt L, Nakaigawa N, et al. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci U S A 1997;94:11445-50. [PubMed]
- 1000 Genomes Project Consortium, Abecasis GR, Auton A, et al. An integrated map of genetic variation from 1,092 human genomes. Nature 2012;491:56-65.
- Peschard P, Park M. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene 2007;26:1276-85. [PubMed]
- Peifer M, Fernandez-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [PubMed]
- Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 2012;44:1111-6. [PubMed]
- Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012;150:1107-20. [PubMed]
- Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012;150:1121-34. [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [PubMed]
- Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 2007;104:20932-7. [PubMed]
- Rong S, Segal S, Anver M, et al. Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci U S A 1994;91:4731-5. [PubMed]
- Mazzone M, Basilico C, Cavassa S, et al. An uncleavable form of pro-scatter factor suppresses tumor growth and dissemination in mice. J Clin Invest 2004;114:1418-32. [PubMed]
- Pilewski JM, Bumbalo TS 3rd, Davis AG, et al. Hepatocyte growth factor promotes tumor growth in a novel in vivo model of human lung cancer. Am J Respir Cell Mol Biol 2001;24:556-62. [PubMed]
- Takayama H, LaRochelle WJ, Sharp R, et al. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci U S A 1997;94:701-6. [PubMed]
- Horiguchi N, Takayama H, Toyoda M, et al. Hepatocyte growth factor promotes hepatocarcinogenesis through c-Met autocrine activation and enhanced angiogenesis in transgenic mice treated with diethylnitrosamine. Oncogene 2002;21:1791-9. [PubMed]
- Santoni-Rugiu E, Nagy P, Jensen MR, et al. Evolution of neoplastic development in the liver of transgenic mice co-expressing c-myc and transforming growth factor-alpha. Am J Pathol 1996;149:407-28. [PubMed]
- Otsuka T, Takayama H, Sharp R, et al. c-Met autocrine activation induces development of malignant melanoma and acquisition of the metastatic phenotype. Cancer Res 1998;58:5157-67. [PubMed]
- Tormo D, Ferrer A, Gaffal E, et al. Rapid growth of invasive metastatic melanoma in carcinogen-treated hepatocyte growth factor/scatter factor-transgenic mice carrying an oncogenic CDK4 mutation. Am J Pathol 2006;169:665-72. [PubMed]
- Krimpenfort P, Quon KC, Mooi WJ, et al. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 2001;413:83-6. [PubMed]
- Sharpless NE, Bardeesy N, Lee KH, et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 2001;413:86-91. [PubMed]
- Jeffers M, Fiscella M, Webb CP, et al. The mutationally activated Met receptor mediates motility and metastasis. Proc Natl Acad Sci U S A 1998;95:14417-22. [PubMed]
- Tward AD, Jones KD, Yant S, et al. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci U S A 2007;104:14771-6. [PubMed]
- Wang R, Ferrell LD, Faouzi S, et al. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol 2001;153:1023-34. [PubMed]
- Zhen Z, Giordano S, Longati P, et al. Structural and functional domains critical for constitutive activation of the HGF-receptor (Met). Oncogene 1994;9:1691-7. [PubMed]
- Amicone L, Spagnoli FM, Spath G, et al. Transgenic expression in the liver of truncated Met blocks apoptosis and permits immortalization of hepatocytes. EMBO J 1997;16:495-503. [PubMed]
- Bhargava M, Joseph A, Knesel J, et al. Scatter factor and hepatocyte growth factor: activities, properties, and mechanism. Cell Growth Differ 1992;3:11-20. [PubMed]
- Rong S, Bodescot M, Blair D, et al. Tumorigenicity of the met proto-oncogene and the gene for hepatocyte growth factor. Mol Cell Biol 1992;12:5152-8. [PubMed]
- Liang TJ, Reid AE, Xavier R, et al. Transgenic expression of tpr-met oncogene leads to development of mammary hyperplasia and tumors. J Clin Invest 1996;97:2872-7. [PubMed]
- Graveel C, Su Y, Koeman J, et al. Activating Met mutations produce unique tumor profiles in mice with selective duplication of the mutant allele. Proc Natl Acad Sci U S A 2004;101:17198-203. [PubMed]
- Graveel CR, London CA, Vande Woude GF. A mouse model of activating Met mutations. Cell cycle 2005;4:518-20. [PubMed]
- Graveel CR, DeGroot JD, Sigler RE, et al. Germline met mutations in mice reveal mutation- and background-associated differences in tumor profiles. PLoS One 2010;5:e13586. [PubMed]
- Ponzo MG, Lesurf R, Petkiewicz S, et al. Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc Natl Acad Sci U S A 2009;106:12903-8. [PubMed]
- Zaffaroni D, Spinola M, Galvan A, et al. Met proto-oncogene juxtamembrane rare variations in mouse and humans: differential effects of Arg and Cys alleles on mouse lung tumorigenesis. Oncogene 2005;24:1084-90. [PubMed]
- Stabile LP, Lyker JS, Land SR, et al. Transgenic mice overexpressing hepatocyte growth factor in the airways show increased susceptibility to lung cancer. Carcinogenesis 2006;27:1547-55. [PubMed]


