Awake video-assisted thoracic surgery in acute infectious pulmonary destruction
Authors’ introduction:
Figure 1 is a picture of the authors team from Department of Thoracic Surgery, City Hospital Nº.1, in Saint-Petersburg, Russia. They are Igor Deynega, Andrey Akopov, Vladimir Egorov, Pavel Ionov from left to right.
Introduction
The history of thoracic surgery started with interventions under local anesthesia. Till the middle of the 60-year of the 20th century almost all lung-related interventions in Russia were done under local anesthesia (1,2).
Later general anesthesia drove out almost completely local one for thoracic surgery use. Surgeons’ confidence in the safety of general anesthesia for patients resulted in considerable surgical advancements and made possible such interventions, which previously seemed to be impossible or fatal. However, as surgery develops new tasks and objectives emerge.
Due to wide routine use of minimally invasive thoracoscopic methods our attitude to local anesthesia is again up for review. Such treatment efficacy indices as hospital stay, surgery costs, drainage duration, etc. are currently highlighted (3-8). More surgeries are done in out-patient settings poorly compatible with general anesthesia. Currently many of thoracic minimally invasive interventions have been proven to be possible without general anesthesia.
Our experience includes video-assisted thoracic surgery (VATS) under local anesthesia in patients with acute infectious pulmonary destructions (AIPD). In developed world the number of such patients is still high (9,10). Their treatment is costly and prolonged and general performance is usually poor limiting chances for “major” surgeries (11). For this patient cohort minimally invasive technologies shortening hospital stay are of paramount importance. Our work presents results of VATS application under local anesthesia and discusses its indications in detail.
Methods
The study was approved by the local Institutional Ethics Committee. All the subjects included in the study gave informed consent.
Our study involved prospective analysis of treatment outcomes for all AIPD patients undergoing VATS under local anesthesia and conscious sedation since January 1, 2010, till December 31, 2013. All interventions were without trachea intubation and epidural anesthesia and the team maintained continuous contact with patients during surgery.
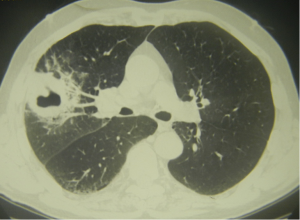
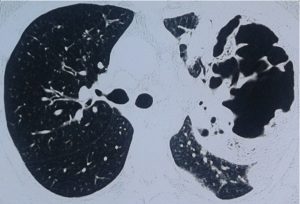
We used classification for AIPD given in ESTS-2014 Textbook of Thoracic Surgery (11). Our study enrolled patients with cavernous AIPD forms—acute purulent abscesses and gangrenous abscesses. Acute purulent abscess develops relatively fast purulent meltdown in lung inflammatory foci forming localized cavity (Figure 2). Gangrenous abscess results in lung tissue necrosis, which along demarcation process turns into a cavity with near-wall or free lung tissue sequestra exhibiting gradual clearance (Figure 3). Patients with these abnormalities underwent non-intubated video abscessoscopy (NIVAS). Destruction cavity at periphery of large size (>5 cm) indicated that in technical sense NIVAS is possible.
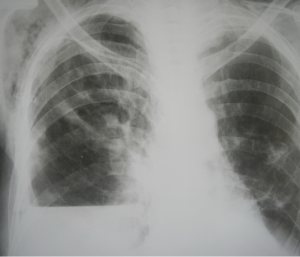
Another pathology when general anesthesia-free surgery was indicated was pyopneumothorax. This term implies lung abscess penetration into patient’s pleural cavity forming bronchial-pleural connection (fistula) and presence of pus (abscess content) and air in his/her pleural cavity (Figure 4). Patients with such complication underwent non-intubated video thoracoscopy (NIVTS).
In this study we did not enroll patients with pleural empyema without destruction cavity in lung tissue.
Indications for NIVAS and NIVTS were as follows: destruction cavity debridement in lungs or pleural cavity, necrotic sequestra removal, differential diagnosis for specific (tuberculosis, cancer) and non-specific destruction causes.
General surgical contraindications were unstable circulation, acute myocardial infarction, acute cerebral circulation disturbances, and coagulation disorders. Special contraindications for interventions under local anesthesia were significant encephalopathy and patient emotional instability.
Prior to interventions all patients were thoroughly examined including assessment of cardiovascular and pulmonary reserves and general patient performance. Testing for clotting system indices was mandatory. Beside general clinical work-up patients underwent chest X-rays and CT, fiber-optic bronchoscopy. Sputum and exudates were sent for cytology and bacteriology testing.
All patients were provided with similar combined conservative treatments of the following main types: transthoracic drainage of purulent pulmonary cavity; transthoracic drainage for pleural cavity in pyopneumothorax cases; antibiotics; fluid replacement. Purulent cavity was drained at the day of admission as the 1st step followed with intensive conservative treatments.
For NIVAS and NIVTS we used Karl Storz endosurgical video-system in a single port mode placing patients in sitting or semi recumbent position to prevent patients from aspiration their purulent cavity content into the healthy lung. Computerized tomography data was used to find closest location to the chest wall. Patients were sedated with intramuscular injection of Diazepam (conscious sedation), up to a maximum dose of 10 mg/1 hour before VATS. Blood pressure and ECG were monitored during surgery. Oxygen was provided via nasal cannulas.
We used infiltrating local anesthesia with 1% xylocaine or 1% lidocaine injecting the solution via thin needle into soft chest tissues from skin to visceral pleura. Then an abscess was tapped—percutaneous needle aspiration was applied. Once pus and air appeared at the tap site a trocar was passed through a 1.5-cm cut. Cavity content was aspirated. Then the cavity was examined with a thoracoscope. Debridement involved application of antiseptic solutions, mechanical fibrin and sequestra removal. We tried to create conditions facilitating free pus outflow post-surgery detaching adhesions that formed purulent cavity compartments and impeded pus outflow. Cavity walls were biopsied in two or three sites. For hemostasis purposes, if necessary, local hemostatic agents were applied. In NIVTS cases patients, if required, underwent partial pulmonary decortication additionally. Drain 24-28-French tubes were placed into caudal cavity parts. Aminocaproic acid solution was introduced via these drainages.
Central venous catheters were placed for all patients. Arterial catheter was not used. Urine catheter was not placed.
In the post-surgery period passive aspiration was employed. Abscess cavity and pleural cavity was daily washed with antiseptic solutions. For periods of massive exudation the cavity was washed 2 or 3 times daily.
No specific analgesia was provided, no narcotic agents were administered.
Results
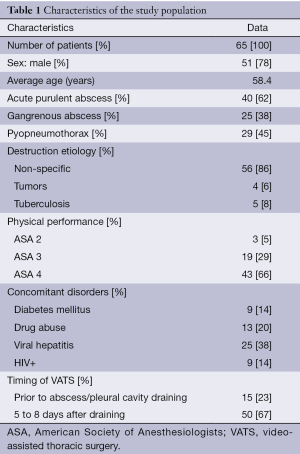
Overall, NIVAS and NIVTS were done for 65 patients. There were 51 men and 14 women. Average patient age was 58.4 (24 to 78). Acute purulent pulmonary abscess was noted in 40 patients (62%), gangrenous one—in 25 patients (38%) (Table 1). Pyopneumothorax caused by abscess breakthrough into a pleural cavity was found in 29 patients (45%).
Full table
In 39 patients (60%) the lesion site was located at the right and in 23 patients (35%)—in the left lung, and 3 patients (5%) had bilateral pulmonary lesions. Mostly lesions were located in posterior upper lobes (31 patients, 48%) and apical segments of lower lobes (25 patients, 38%). In more than a half of the patients pulmonary destruction cavity exceeded 10 cm in the largest diameter, while in 21 of them (32%) the purulent lesion size exceeded 15 cm.
At the time of the admission to hospital American Society of Anesthesiologists (ASA) physical status class were the following: I (none), II (3 patients), III (19 patients), and IV (43 patients). The most severe condition was observed in patients with gangrenous abscesses (ASA status IV—21 of 25 patients, 84%) as well as in patients with pyopneumothorax (22 of 29, 76%). Major symptoms were purulent sputum in large amounts of above 100 mL per day and fever in all patients, dyspnea of Modified Medical Research Council (MMRC) Grade 3 to 4—in 49 of 65 patients (75%) as well as fatigue, wasting syndrome, hypotension and tachycardia.
Severe pulmonary disorders (COPD, asthma) were found in 20 patients (31%), coronary failure—in 21 patients (32%), diabetes mellitus—in 9 patients (14%), HIV—in 9 patients (14%), hepatitis B and C—in 28 patients (38%). A total of 46 patients (71%) were current smokers and 13 patients (20%) were drug users.
NIVAS was done in 36 patients (55%), NIVTS—in 29 patients (45%). Six patients with gangrenous abscesses and three ones with pyopneumothorax underwent two surgeries, i.e., in total, enrolled patients had 42 NIVAS and 32 NIVTS interventions. Mean NIVAS time was 11.5 min (range, 7-15 min), NIVTS—13.4 min (range, 10-17 min).
NIVAS description
Purulent abscesses (11 patients) typically presented excessive granulation tissue, massive fibrin deposits; destruction cavity usually was round-shaped. Manipulations in purulent abscess cases were mostly related to biopsy and cavity wall treatment removing fibrin, purulent and necrotic tissue. However, we did not notice prominent adhesions separating such purulent cavity and impeding pus outflow. Gangrenous abscesses (25 patients) presented with an irregular shape cavity, separating adhesions and distinct, hard-to-drain pockets. The cavity walls were usually uneven, covered with necrotic or fibrin covered sites as well as areas of “naked” lung tissue. Its content was of soot-colored, thick, often with foul smell. Near-wall or free locating sequestra were present. In case of NIVAS interventions possibility to remove sequestra and to cut adhesions segregating such purulent cavities was especially important. These manoeuvres are the base for therapeutic NIVAS effects in gangrenous abscess patients.
NIVTS description
Typically there was a large cavity with massive depositions of fibrin easy to remove by simple mechanical curettage. Frequent finding was adhesions segregating the pleural cavity. It was not always easy to locate a lung-pleural fistula. Entire set of VATS manipulations is shown in the Table 2.
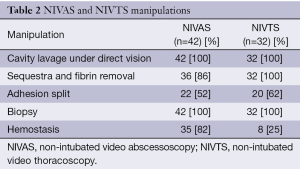
Full table
In none of the cases we saw intrasurgical complications. Also none of the patients required trachea intubation. In none of our cases with conversion to thoracotomy was required. Patients did not present considerable fear of NIVAS and NIVTS under local anesthesia.
Post-surgical complications developed after 11 interventions (13%) (Table 3). The bleeding was caused by an attempt of fixed sequestra removal and was stopped with non-surgical methods. Subcutaneous emphysema of chest wall disappeared without treatment. Pneumothorax treatment involved pleural tube placement. In three patients with chest wall phlegmon soft tissues were cut to rib carcass followed with daily dressing replacement and wound debridement. The process was eventually eliminated in two of three patients. One patient died due to the main disease progression.
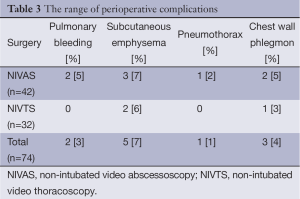
Full table
Timing of NIVAS and NIVTS procedures was found to be of paramount importance for ensuring complete therapeutic effectiveness. In 15 patients NIVAS and NIVTS were done immediately prior to abscess/pleural cavity draining, while in other 50—within 5 to 8 days, after pus was removed and acute inflammatory signs subsided. However, in 5 of 15 (33%) cases operated prior to draining we were unable to implement our surgical plan to its full extent and to provide comprehensive cavity debridement and biopsy. Therefore, later these patients required another surgery. Moreover, both pulmonary bleeding episodes and all three cases of chest wall phlegmon took place in this patient group. In patients with post-draining interventions we managed to fulfill our pre-surgery plans in 91% with no severe complications. Biopsy data was also found to be of higher value in patients with post-draining surgery. In two patients where histology material after first surgery was found to be of poor quality another biopsy at the second NIVAS enabled us to diagnose tuberculosis (one case) and cancer (one case).
Within the 30-day post-surgery one patient with gangrenous abscess died due to inflammation progression and increasing intoxication. Despite his destruction cavity was sufficiently drained we did not succeed in controlling the infectious process in lung tissue caused by pseudomonas aeruginosa and anaerobic microbes due to multiple antibiotic resistance.
Discussion
The grounds for NIVTS and NIVAS under local anesthesia in patients with AIPD were as follows: first, short time of the intervention and, second, rather small range of manipulations to be done during the surgery. Additional rationale for local anesthesia use in AIPD patients is no need for separate contralateral lung ventilation.
The main advantage of the local anesthesia approach according to our results is the method safety. During the surgery regulatory and adaptive central nervous mechanisms still function, thus, respiration and circulation changes during the interventions are adequately compensated (12,13). Also it is important that patients with AIPD are able to cough out their bronchial content as much as possible. Effective coughing and sitting position for interventions prevented infected bronchial content from finding a way into patient’s healthy lung. In turn, it diminishes atelectasis risks, which is one of the causes of post-surgical pneumonia. Such complication has never been seen in any of our clinical cases.
During surgery our patients did not complain of considerable discomfort. In general, the method in technical sense is not extremely sophisticated and for majority of thoracic surgeons its learning curve is not steep. Curiously, our anesthetist attitude to local anesthesia interventions—after sufficient experience was gained—became positive. Currently general anesthesia with trachea intubation in patients with infectious pulmonary destructions is only for more traumatic interventions such as full decortication or resections. Other researchers in the field of general anesthesia-free thoracoscopy showed less surgery time, shorter hospital stay and lower treatment costs (3,5,6,14,15). We did not compare cost and duration for VATS-treated patients because since 2009 all thoracoscopic manipulations in AIPD cases were done under local anesthesia and without tracheal intubation. The most important factors when choosing the discussed approach is less operating theater utilization, lower hospital staff involvement, elimination of trachea intubation and related additional risks. One should take into account that this particular patient cohort is different from the general patient population. They are usually of lower social status and their microbial gamut is represented with highly drug resistant bacteria (11,16). AIPD patients should be treated in in-patient settings of specialized units. Every day of their hospital stay implies rather high costs and expenditures. With the aforementioned in mind one can see that applying minimally invasive technologies in such patients is of paramount importance. Also it is quite valuable that such patients have positive attitude to interventions under local anesthesia.
Taking into account the previous discussion and our clinical experience we managed to articulate main indications for NIVAS and NIVTS in AIPD patients. First, such surgery is always both diagnostic and therapeutic. Second, NIVAS and NIVTS interventions should always follow transthoracic draining, pus removal and inflammation subsidence. Although it appears that it is impossible to avoid repeated NIVAS completely. Repeated manipulations are required since there are two types of sequestra—free and fixed or near-wall ones. Attempting for rough removal of the latter might end up in severe bleeding. The question of how to determine if such sequestra are free or fixed and, thus, their removal is fraught with grave consequence is one of paramount importance. And this issue defines time frame for both primary and repeated NIVAS procedures. However, at the moment, regretfully, there is no proper final solution present.
Conclusions
In conclusion, we need to stress that NIVAS and NIVTS under local anesthesia and sedation are well tolerated by patients, safe and should be used more often in AIPD cases. Timing of NIVAS and NIVTS procedures was found to be of paramount importance for ensuring complete therapeutic effectiveness. It is necessary to gain more experience and to promote such interventions to be done in other thoracic surgery clinics to produce evidence-based guidelines for this method and its applications.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bogush LK. Development of surgery of pulmonary tuberculosis in the Soviet Union for the last 50 years. Probl Tuberk 1967;45:3-9. [PubMed]
- Grigorian AV, Lokhvitskii SV. Major results and prospects for the development of lung surgery in the USSR. Grudn Khir 1972;14:14-21. [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [PubMed]
- Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg 2007;32:346-50. [PubMed]
- Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. [PubMed]
- Pompeo E, Mineo TC. Awake pulmonary metastasectomy. J Thorac Cardiovasc Surg 2007;133:960-6. [PubMed]
- Katlic MR, Facktor MA. Video-assisted thoracic surgery utilizing local anesthesia and sedation: 384 consecutive cases. Ann Thorac Surg 2010;90:240-5. [PubMed]
- Lesser TG. Laser application enables awake thoracoscopic resection of pulmonary nodules with minimal access. Surg Endosc 2012;26:1181-6. [PubMed]
- Pagès PB, Bernard A. Lung abscess and necrotizing pneumonia: chest tube insertion or surgery? Rev Pneumol Clin 2012;68:84-90. [PubMed]
- Deĭnega IV, Egorov VI, Ionov PM, et al. Diagnostics and surgical treatment of lung cancer in conditions of special thoracal department for patients with purulent lung diseases. Vestn Khir Im I I Grek 2014;173:15-8. [PubMed]
- Akopov A, Egorov V, Furák J. Bacterial Lung Infections. In: Kuzdzal J. eds. ESTS textbook of thoracic surgery. Cracow: Medicina Praktyczna, 2014:517-27. Available online: http://www.ests.org/textbook/default.aspx
- Ambrogi MC, Fanucchi O, Gemignani R, et al. Video-assisted thoracoscopic surgery with spontaneous breathing laryngeal mask anesthesia: preliminary experience. J Thorac Cardiovasc Surg 2012;144:514-5. [PubMed]
- Kiss G, Claret A, Desbordes J, et al. Thoracic epidural anaesthesia for awake thoracic surgery in severely dyspnoeic patients excluded from general anaesthesia. Interact Cardiovasc Thorac Surg 2014;19:816-23. [PubMed]
- Hazelrigg SR, Nunchuck SK, LoCicero J 3rd. Video Assisted Thoracic Surgery Study Group data. Ann Thorac Surg 1993;56:1039-43; discussion 1043-4. [PubMed]
- Pompeo E. State of the art and perspectives in non-intubated thoracic surgery. Ann Transl Med 2014;2:106. [PubMed]
- Schweigert M, Dubecz A, Beron M, et al. Surgical therapy for necrotizing pneumonia and lung gangrene. Thorac Cardiovasc Surg 2013;61:636-41. [PubMed]