
Progesterone protocol versus gonadotropin-releasing hormone antagonist protocol in women with polycystic ovarian syndrome undergoing in vitro fertilization treatments with frozen-thawed embryo transfer: a prospective randomized controlled trial
Introduction
Patients with polycystic ovary syndrome (PCOS) are at high risk of ovarian hyperstimulation syndrome (OHSS) during in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) treatment (1,2). Increasing evidence has confirmed that frozen-embryo transfer (FET) is related to a lower risk of OHSS (3). A recent large, multi-center randomized trial performed by Chinese researchers demonstrated that FET resulted in a lower risk of OHSS and a higher rate of live birth than fresh embryo transfer in women with PCOS (4). The ‘‘freeze-all’’ strategy is recommended for PCOS patients to reduce the potential of moderate/severe OHSS. Meanwhile, our team firstly introduced the usage of exogenous progestational agents as an alternative pituitary modulator for the prevention of premature luteinizing hormone (LH) surges in ovarian stimulation (5-9). Until now, limited data are available regarding the use of the progesterone protocol in PCOS patients. Therefore, we performed a prospective randomized controlled trial to explore the differences between progestins and gonadotropin-releasing hormone antagonists (GnRH-ant) during ovarian stimulation in PCOS patients. The hormone dynamics, ovarian response, embryological performance, and pregnancy outcomes following FET of a progesterone protocol and GnRH-ant protocol group treated with IVF/ICSI were compared.
We present the following article in accordance with the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1592).
Methods
Study design and participants
This randomized controlled study was performed in the Department of Assisted Reproduction of the Ninth People’s Hospital affiliated to the Shanghai Jiao Tong University School of Medicine, with the approval of the Institutional Review Board of the Ninth People's Hospital (number: 2015-54). The study was conducted according to the Declaration of Helsinki (as revised in 2013). Patients undergoing their first ovarian stimulation cycle between July 2015 and December 2018 were recruited and signed informed consent relevant to infertility treatment with IVF/ICSI procedures was obtained.
The diagnosis of PCOS was made according to the 2003 Rotterdam criteria. Patients under 38-year of age, with a BMI <28 kg/m2, and planning to be treated with IVF/ICSI with the “freeze-all” strategy were eligible to participate. Those with a previous IVF/ICSI history, severe endometriosis (grade 3 or higher), significant systemic disease, or other situations which rendered them unsuitable for ovarian stimulation or the receipt of hormonal drugs in the past three months were excluded from the study.
Study protocol
The study group received soft progesterone capsules (brand name: Utrogestan, 100 mg, Laboratories Besins International, France) 100 mg and 150 IU of human menopausal gonadotropin (hMG) concomitantly from the menstrual cycle (MC) day 3 until the trigger day. The control group received the GnRH-ant protocol consisting of HMG 150 IU’s daily injection from MC 3 until the trigger day. GnRH-ant (Cetrotide, 0.25 mg, Merck-Serono) was started when at least one of the following criteria were met: LH >10 IU/L, the presence of at least one follicle with mean diameter >14 mm, or serum E2 level >600 pg/mL. According to follicular development, follicle size, and serum hormone levels, the dose of hMG remained fixed for the first 5–6 days of ovarian stimulation and could be adjusted after that. Once three dominant follicles reached 18mm in diameter, 0.1 mg GnRH-a (Decapeptyl®; Ferring Pharmaceuticals, Germany) was administered to induce oocyte maturation oocyte retrieval was performed 36–38 hours after trigger. In vitro fertilization, embryo culture, and assessment were carried out based on routine procedures in our clinic (5-9). Patients in both groups used a similar protocol for endometrial preparation, consisting of hormone replacement therapy or mild stimulation using Letrozole with or without hMG (10).
Randomization and sample size estimate
A previous study of ours showed that the mean number of oocytes retrieved from PCOS patients undergoing ovarian stimulation using the progesterone protocol was 13, with a standard deviation of seven. Based on this, we concluded a sample size of 59 in each group would yield 85% power and establish non-inferiority at the 10.0% level of significance to detect a significant difference of three in the number of oocytes retrieved. Given the possibility of dropouts, we designed the study to include a total of 60 women in each group.
Patients who met the eligibility were randomly allocated to the study group or the control group at a ratio of 1:1 using a computer-generated random number. While patients were not blinded to the group assignment, the physicians and embryologists involved in the procedures of oocyte retrieval and embryo transfer were blinded to the treatment assignments in the trial.
Hormone analysis
Serum follicle stimulating hormone (FSH), LH, estradiol (E2), and progesterone (P) concentrations were measured with the use of a Chemiluminescent method (Abbott Biologicals B.V., the Netherlands). The upper limit for E2 measurement was 5,000 pg/mL, and the lower limits of sensitivity for FSH, LH, E2, and P were 0.06 IU/L, 0.09 IU/L, 10 pg/mL, and 0.1 ng/mL, respectively (5-8).
Outcome measures
The primary outcome measure was the number of oocytes retrieved. Secondary outcome measures included the occurrence of premature LH rise/surge, duration and dosage of hMG, the number of follicles with diameter >10/14 mm on the trigger day, mature oocytes and viable embryos, the moderate/severe OHSS rate, the clinical pregnancy rate, implantation rate, miscarriage rate, and live birth rate. A premature LH rise was defined as a serum LH ≥10 IU/L. A premature LH rise accompanied by a P rise (>2 ng/mL) was defined as a premature LH surge in the GnRH-ant protocol, while the definition of a premature LH surge in the progesterone protocol was an LH ≥10 IU/L on the trigger day.
Statistical analysis
An intention-to-treat approach was utilized in the statistical analysis. Continuous variables were presented as mean ± standard deviation (SD), while categorical variables were shown as number and percentage. The Student’s t-test was used for normally or near-normally distributed data, while the Mann-Whitney U test was employed for non-normally distributed data to compare continuous variables. Categoric variables adopted the Chi-square test and Fischer’s exact test for comparisons. Two-sided P<0.05 was considered as statistical significance. The Statistical Package for the Social Sciences for Windows version 24.0 was used for analysis (SPSS, Chicago, IL, USA).
Results
Patient characteristics
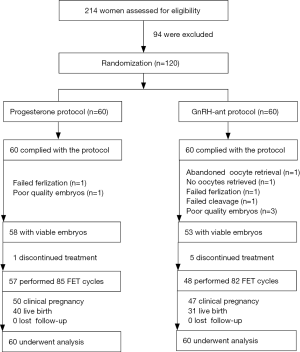
Figure 1 shows the flowchart of the study. A total of 120 women meeting the eligibility criteria were randomly allocated to undergo the progesterone protocol (the study group) or GnRH-ant protocol (the control group). All patients in the study group completed oocyte retrieval, of which 58 obtained viable embryos. In contrast, one participant in the control group canceled the oocyte retrieval for personal reasons, and 53 obtained viable embryos. During the follow-up period, 57 patients finished 85 FET cycles in the study group, whereas 82 FET cycles were performed in 48 participants in the control group.
The general characteristics of the patients are presented in Table 1. The age, BMI, duration of infertility, the distribution of IVF indication, basal FSH, LH, and P were similar in the two groups. The basal E2 was significantly higher in the GnRH antagonist group (P<0.05), with no clinical significance.
Ovarian stimulation and embryo results
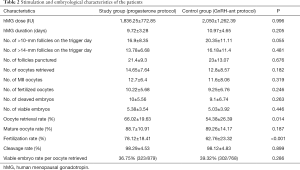
Table 2 shows the ovarian stimulation and embryo results of the two groups. The GnRH-ant duration was 3.6±2.1 days in the control group. The total hMG dose (1,836.25±772.85 versus 2,050±1,262.39 IU) was slightly less, and the mean hMG duration (9.72±3.28 versus 10.97±4.65) was shorter in the study group when compared to the control group, with no significant difference (P>0.05). In the study group, the number of follicles with a diameter >10 mm (16.9±8.35 versus 20.35±11.11) and >14 mm (13.78±6.68 versus 16.18±11.4) were less than those in the control group but did not reach significance (P>0.05). The number of retrieved oocytes was 14.65±7.64 in the study group and 12.8±8.57 in the control group (P>0.05). The two groups were comparable regarding the number of mature oocytes, fertilized oocytes, cleaved embryos, and viable embryos (P>0.05). Similarly, no between-group difference was found in the viable embryo rate per oocyte retrieved (P>0.05). However, the oocyte retrieval rate (66.02%±19.63% versus 54.38%±26.39%) and fertilization rate (78.12%±18.41% versus 62.76%±23.32%) were significantly more in the study group when compared to the control group (P>0.05). None of the patients experienced moderate/severe OHSS during the study.
Full table
Pregnancy outcomes following frozen-thawed embryo transfer
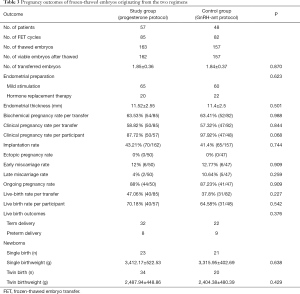
Table 3 illustrates pregnancy outcomes in the two groups. In this study, a total of 167 FET cycles were completed in 105 patients until August 2019. One patient in the study group and six in the control group who had viable embryos did not complete their FET cycles for personal reasons. The clinical pregnancy rate per transfer in the study group was comparable to that in the control group [58.82% versus 57.32%, RR 0.94 (95% CI: 0.508, 1.738), P>0.05], and the implantation rate was similar between the two groups [43.21% versus 41.4%, RR 0.929 (95% CI: 0.595, 1.448), P>0.05]. The early miscarriage rate showed no significant difference in both groups [12% versus 12.77%, RR 1.073 (95% CI: 0.32, 3.595), P>0.05], and the live birth rate per transfer was slightly higher in the study group than that in the control group [47.06% versus 37.8%, RR 0.684 (95% CI: 0.369, 1.267), P>0.05], without significant differences. There were no statistically significant differences in the birthweights of singleton and twin new-borns.
Full table
Dynamic hormone profile during ovarian stimulation
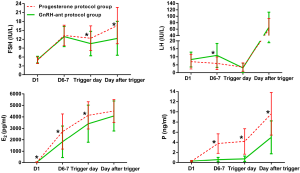
Figure 2 shows the dynamic hormone profile during ovarian stimulation in the two groups. The starting day of ovarian stimulation was denoted as day 1 (D1). FSH levels on the trigger day and the day after were higher in the study group than those in the control group (P<0.05). On D6-7 and the trigger day, the average E2 was lower in the control group (P<0.05), then it increased to be comparable with that of the study group on the day after trigger (P>0.05). The serum P level in the study group was higher than that in the control group throughout the process of ovarian stimulation (P>0.05).
An elevated basal LH level (LH ≥10 IU/L) was detected in sixteen patients of the study group (26.67%), with a range between 10.01 and 18.96 IU/L, and eighteen patients of the control group (30%), ranging from 10.05 to 21.51 IU/L. The mean serum LH value was higher on D6-7 in the control group than in the study group (7.47±0.97 versus 3.98±0.52 IU/L, P<0.05). No between-group differences were found in the two groups in the LH level on the trigger day and the day after (P>0.05). Premature LH surges were not detected in any patient. A suboptimal pituitary response after trigger (LH <15 IU/L) was found in one study group case (12.35 IU/L) and control group case (11.48 IU/L), although both obtained component oocytes and embryos and successfully conceived with FET.
Before the first GnRH-ant administration, 27 women (45%) had an LH value ≥10 IU/L on D6-7 in the control group, of which 13 patients were found with elevated basal LH levels (LH value ≥10 IU/L). Concomitant rises of serum progesterone value >2 ng/mL on D6-7 were not found in any control group patient. After GnRH-ant initiation, endogenous LH levels were effectively cut down in 26 women, with the LH value ranging from 0.92 to 7.13 IU/L on the trigger day. However, in one case, the LH value was not suppressed by GnRH-ant administration. The LH values in this patient were 13.97 IU/L on D1, 17.06 IU/L on D7, and 16.09 IU/L on the trigger day, and the serum progesterone did not rise (>2 ng/mL). In the study group, LH ≥10 IU/L on D6-7 was observed in eight patients (13.33%), of which elevated basal LH levels were seen in five. Nevertheless, the LH level in those patients with premature LH rise was gradually reduced, with a value ranging from 1.65 to 7.27 IU/L on the trigger day.
Discussion
The definition of a premature LH surge has been based primarily on the incidence of early LH rise and serum progesterone levels. The threshold level of LH rise was reported to be 10 IU/L (11), 12.4 IU/L (12), or 15 IU/L (13), while the cut-off level of progesterone ranged between 1 and 2.25 ng/mL (11-14). In our study, a premature LH surge was defined as a premature LH rise ≥10 IU/L accompanied by P rise (>2 ng/mL) in the GnRH-ant protocol. Serum progesterone level could not be considered as an indicator for a premature LH surge in the progesterone protocol since oral delivery of soft progesterone capsules (Utrogestan) may interfere with the measurement of endogenous P production.
The mechanism of LH suppression using GnRH-ant or progestin is distinct. GnRH-ant administration could lead to rapid suppression of pituitary LH secretion by competitively blocking the GnRH receptor, with an obvious dose-dependent suppression effect. In contrast, the inhibition of LH levels with progestin is indirect by regulating the hypothalamus GnRH secretion and requires sufficient duration before estrogen priming (5). The variable absorption ability of progestin differs between individuals, and serum E2 concentration may exert a synergistic effect with progestin in LH suppression (6-9). A dose-finding study of the Utrogestan showed there was no direct relation between Utrogestan dosages and different endogenous LH levels (7). Taken together, progestin could not induce an immediate LH suppression in the mode of GnRH-ant. A recent retrospective cohort study by Japanese researchers showed progestin applied in the method of a flexible GnRH-ant protocol obtained a similar number of metaphase II oocytes and pregnancy rates compared to a flexible GnRH-ant protocol, and no premature ovulations were detected (15). Nevertheless, a major limitation of that study was that serum LH levels were not monitored during ovarian stimulation, so whether there were premature LH rises or surges could not be distinguished.
A premature LH rise (≥10 IU/L), which precedes an LH surge, is usually used as a predictor for pituitary modulators' administration. Our data showed the incidence of premature LH rise in the two groups was significantly different (13.33% versus 45%, P<0.05), which may be attributed to different patterns of LH suppression with progestin and GnRH-ant. In our previously published retrospective study on PCOS patients using the progesterone protocol, eight patients out of 123 experienced premature LH rises (6), which was in line with the present study results. However, the percentage of patients with premature LH rise in the GnRH-ant protocol was relatively higher than previously reported (1.4–38.8%) (11,12,15). All of the premature LH rises occurred before GnRH-ant administration in our study, which was following literature reporting that around 80% of these surges occurred before the start of GnRH-ant treatment (11,12,15,16). Some researchers deem that GnRH-ant's fixed initiation on day 6 for the prevention of earlier LH rises is likely to be too late (16). We started GnRH-ant from the sixth day of ovarian stimulation or later, which was one reason for the high rate of premature LH rises. Also, rapid follicular growth in PCOS patients may also account for the abrupt increase of LH; as previously described earlier, LH rises were often seen in high responders (11,12,15,16).
Moreover, an elevated basal LH level was detected in 48.15% of patients, which was closely related to the high rate of premature LH rise. Some data suggests that high basal LH levels are associated with impaired fertilization and pregnancy rates and higher miscarriage rates (17). Our retrospective data analysis, including 1011 PCOS patients, has shown that 105 patients with LH levels ≥10 mIU/mL obtained a higher number of oocytes retrieved, mature oocytes, and top-quality embryos than those with lower basal LH levels (18). We routinely initiate ovarian stimulation without controlling the high basal LH level of PCOS patients in our clinic, which may contribute to the high rate of basal LH value ≥10 IU/L (28.33%) seen in this trial.
The gradual suppression of pituitary LH secretion with progestins causes serum LH values during ovarian stimulation to decline slowly and maintain relatively steady. This differs from the rapid decline of LH values using GnRH-ant. In a dose-finding study of GnRH-ant, authors demonstrated that large changes in LH levels (either an increase or decrease from basal levels) are associated with a decreased chance of clinical pregnancy (12). One of the possible reasons presented by the authors was that violent fluctuation in LH levels may interfere with the incorrect sequence of maturational changes and synchronization between nuclear and cytoplasmic maturation, along with advanced endometrium maturation (12). In our study, all patients underwent FET, which could avoid the effect of LH changes on the endometrium. Our data showed no between-group differences in clinical pregnancy and implantation rates.
In contrast, the number of oocytes retrieved, MII oocytes, fertilized oocytes, cleaved embryos, and viable embryos in the progesterone protocol were greater than those of the GnRH-ant protocol, despite there being no statistical significances. Moreover, the oocyte retrieval and fertilization rates were significantly higher in the progesterone protocol. Additionally, no moderate/severe OHSS was observed in our study. Except for the “freeze-all" strategy, other prevention strategies, including vaginal delivery, use of the dopamine agonist cabergoline, and the application of GnRH agonist instead of human chorionic gonadotropin for the trigger, also play a role in the prevention of OHSS (6). These results confirmed that the progesterone protocol could be provided as a feasible alternative regimen in PCOS patients during IVF/ICSI treatments with embryo cryopreservation.
The results of this should be interpreted with caution due to its limited sample size and because the physiological basis for the association between the different patterns of LH suppression with GnRH-ant or progestin and clinical outcomes requires further exploration in basic experimental studies. This study also used a freeze-all strategy that does not reflect real-world status because fresh embryo transfer is an option for patients with GnRH-ant in practical procedures. The progesterone protocol in PCOS patients should also take the increased economic burden of embryo cryopreservation into account, and cost-effective analysis to evaluate all options for patients is needed.
In conclusion, our data show that the progesterone protocol is comparable with the GnRH-ant protocol regarding oocytes/embryo yields and the probability of clinical pregnancy, but the two regimens are distinct in the regulation of pituitary LH secretion.
Acknowledgments
The authors express sincere thanks to all patients and staff of our Hospital for their contribution.
Funding: The Science and Technology Commission of Shanghai Municipality (grant number: 18411963800) and the Shanghai First Maternity and Infant Hospital affiliated to Tongji University School of Medicine (grant number 2020RC02) funded the present study.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1592
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1592
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1592). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Human Ethics Committee approval of this study was obtained by the Institutional Review Board (IRB) of the Shanghai Ninth People’s Hospital on 6 July 2015 (number: 2015-54). All patients have signed informed consent for this research. Trial registration: Chinese clinical trial registration number: ChiCTR-IOR-15006633 (http://www.chictr.org.cn/edit.aspx?pid=11261&htm=4). All patients have signed informed consent for this research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19-25. [Crossref]
- Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update 2002;8:559-77. [Crossref] [PubMed]
- Chen ZJ, Shi Y, Sun Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med 2016;375:523-33. [Crossref] [PubMed]
- Shi Y, Sun Y, Hao C, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med 2018;378:126-36. [Crossref] [PubMed]
- Zhu X, Zhang X, Fu Y. Utrogestan as an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Medicine (Baltimore) 2015;94:e909. [Crossref] [PubMed]
- Zhu X, Ye H, Fu Y. The Utrogestan and hMG protocol in patients with polycystic ovarian syndrome undergoing controlled ovarian hyperstimulation during IVF/ICSI treatments. Medicine (Baltimore) 2016;95:e4193. [Crossref] [PubMed]
- Zhu X, Ye H, Fu Y. Use of Utrogestan during controlled ovarian hyperstimulation in normally ovulating women undergoing in vitro fertilization or intracytoplasmic sperm injection treatments in combination with a “freeze all” strategy: a randomized controlled dose-finding study of 100 mg versus 200 mg. Fertil Steril 2017;107:379-86.e4. [Crossref] [PubMed]
- Zhu X, Ye H, Fu Y. Duphaston and human menopausal gonadotropin protocol in normally ovulatory women undergoing controlled ovarian hyperstimulation during in vitro fertilization/intracytoplasmic sperm injection treatments in combination with embryo cryopreservation. Fertil Steril 2017;108:505-12.e2. [Crossref] [PubMed]
- Zhu X, Ye H, Fu Y. Comparison of neonatal outcomes following progesterone use during ovarian stimulation with frozen-thawed embryo transfer. Sci Rep 2017;7:7835. [Crossref] [PubMed]
- Zhang J, Liu H, Wang Y, et al. Letrozole use during frozen embryo transfer cycles in women with polycystic ovary syndrome. Fertil Steril 2019;112:371-7. [Crossref] [PubMed]
- Messinis IE, Loutradis D, Domali E, et al. Alternate day and daily administration of GnRH antagonist may prevent premature luteinization to a similar extent during FSH treatment. Hum Reprod 2005;20:3192-7. [Crossref] [PubMed]
- Huirne JA, van Loenen AC, Schats R, et al. Dose-finding study of daily GnRH antagonist for the prevention of premature LH surges in IVF/ICSI patients: optimal changes in LH and progesterone for clinical pregnancy. Hum Reprod 2005;20:359-67. [Crossref] [PubMed]
- Chen Q, Chai W, Wang Y, et al. Progestin vs. gonadotropin-releasing hormone antagonist for the prevention of premature luteinizing hormone surges in poor responders undergoing in vitro fertilization treatment: a Randomized controlled trial. Front Endocrinol (Lausanne) 2019;10:796. [Crossref] [PubMed]
- Xu B, Li Z, Zhang H, et al. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril 2012;97:1321-7. [Crossref] [PubMed]
- The Ganirelix Dose-finding Study Group. A double-blind, randomized, dose-finding study to assess the efficacy of the gonadotrophin-releasing hormone antagonist ganirelix (Org 37462) to prevent premature luteinizing hormone surges in women undergoing ovarian stimulation with recombinant follicle stimulating hormone (Puregon). The ganirelix dose-finding study group. Hum Reprod 1998;13:3023-31. [Crossref] [PubMed]
- Kolibianakis EM, Venetis CA, Kalogeropoulou L, et al. Fixed versus flexible gonadotropin releasing hormone antagonist administration in in vitro fertilization: a randomized controlled trial. Fertil Steril 2011;95:558-62. [Crossref] [PubMed]
- Kolibianakis EM, Albano C, Kahn J, et al. Exposure to high levels of luteinizing hormone and estradiol in the early follicular phase of gonadotropin-releasing hormone antagonist cycles is associated with a reduced chance of pregnancy. Fertil Steril 2003;79:873-80. [Crossref] [PubMed]
- Sun L, Ye J, Wang Y, et al. Elevated basal luteinizing hormone does not impair the outcome of human menopausal gonadotropin and medroxyprogesterone acetate treatment cycles. Sci Rep 2018;8:13835. [Crossref] [PubMed]
(English Language Editors: B. Draper and J. Gray)



