Comparison of different stimulation protocols used in in vitro fertilization: a review
Introduction
Controlled ovarian hyperstimulation (COH) is a fundamental step of in vitro fertilization (IVF) that has been in practice since its initial practice in the 1970s (1). Over time IVF or assisted reproduction techniques have evolved to fulfill the needs of patients who range from low, intermediate and high responders. The discoveries of gonadotropin-releasing hormone (GnRH) analogues and inhibitors of natural steroid hormone (oestradiol) such as clomiphene citrate (CC) have offered multiple options in terms of assisted reproduction, and have improved IVF success rates (2,3). Studies have continued to publish numerous regimens for ovarian stimulation including GnRH analogues, CC and gonadotropin mixtures (Gn), or the combination therapy regimens consisting of CC, recombinant follicle stimulating hormone (FSH) and luteinizing hormone (LH) (1,4). Depending on the usage of a GnRH agonist versus antagonist analogue, GnRH analogue IVF protocols are classified as GnRH agonist or GnRH antagonist protocols. Another protocol utilizes the usage of CC in combination with Gn or FSH, which is termed a minimal stimulation protocol (1,4-6). This review compares the advantages and disadvantages of each of these three protocols with respect to IVF or assisted reproduction.
GnRH agonist long protocol and antagonist protocol
GnRH agonist and antagonist protocols utilize agonistic or antagonistic analogues of GnRH. GnRH analogues are decapeptides designed after human GnRH in order to interact with GnRH receptors. These analogues have certain amino acids substitutions in the gonadotropin amino acid sequence that increases the half-lives and competencies of analogues compared to natural hormones (2,7,8). GnRH agonists allows sustained stimulation of gonadotropin secretion, while GnRH antagonists act as mediators of chemical hypophysectomy (9). Overall, both analogues are widely used in IVF to induce folliculogenesis via prevention of endogenous LH surge and timed oocyte retrieval (10-12). Several agonistic analogues (triptorelin, leuprorelin, deslorelin, goserelin and nafarelin) and a couple antagonistic analogues (cetrorelix and ganirelix) have been introduced into clinical practices (9). Among the various GnRH agonist long protocols, namely ultra short, short and long, the long GnRH agonist protocol has been used as the gold standard in IVF since its discovery in the 1980s (10,13). The recent development of GnRH antagonists has offered an alternative approach in IVF treatment.
The GnRH long agonist protocol (Figure 1) starts with administration of 0.1 mg GnRH agonist (e.g., triptorelin) on cycle-day 21 followed by administration of gonadotropin at 150-225 international units (IU) daily starting on cycle-day 2. The adjustment of gonadotropin dose is based on follicular development. Continual administration of GnRH agonist and gonadotropin lasts until the start of human chorionic gonadotropin (HCG) injection, which is approximately 14 days post GnRH agonist regimen or when follicles reached from 16 to 18 millimeters (mm) in size. For the GnRH antagonist protocol (Figure 1), administration of gonadotropin at 150-225 IU daily is initiated after monitoring of patients’ follicles sizes on cycle-day 2/3. Gonadotropin dosage varies according to the follicular response. Approximately after the 6th days of gonadotropin injection or when follicular size reaches more than or equal to 14 mm, subcutaneous administration of the GnRH antagonist (e.g., cetrorelix) begins. In both protocols, there is routine monitoring of patients via trans-vaginal sonography (TVS) and hormonal profiling of FSH, LH, estrogen and progesterone levels of patients. After 34-36 h of HCG injection the mature oocytes are retrieved. Patients with increasing LH and estrogen levels are at risk for premature ovulation and require additional monitoring. It is important to cease the cycles if there is added risk of ovarian hyper stimulation syndrome (OHSS).
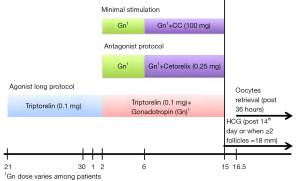
Minimal stimulation protocol
CC is an estrogen receptor modulator and a competitive inhibitor of oestradiol, which has been used for fertility treatment since the last four decades (3). The anti-estrogenic property of CC is the main drawback of this treatment. However, it was later discovered that the antiestrogenic property may cause suppression of the premature LH surge that is responsible for maintaining folliculogenesis (4). Minimal stimulation protocol utilizes CC in conjunction with human menopausal gonadotropin (HMG), which is more effective compared to administering HMG alone (46% vs. 25.9%) (3,4,14). In this treatment protocol (Figure 1), administration of CC occurs on the 6th day, or earlier depending on LH level rise, and continues until HCG administration. This is followed by the retrieval of mature oocyte and IVF.
Letrozole, an aromatase inhibitor is used alternative to clomiphene for minimal stimulation protocol in some clomiphene resistant patients. Letrozole is used at 2.5 mg starting on day 2 or 3 of menstruation for 5 days in conjunction with gonadotropin. However, letrozole was developed to treat metastatic breast cancer and is still not approved for use in ovulation induction.
Criteria for IVF protocol selection
The use of long agonist protocol, antagonist protocol or minimal stimulation protocol on each patient is usually based on the physician’s decision. The decision is based on the benefits and shortcomings of each treatment option, and most importantly on the patients’ response. Gonadotropin stimulation patients fall under three categories based on their response: (I) high responders; (II) intermediate responders and (III) poor responders (15,16). Most commonly, FSH level, oocyte number, cycle cancellation rate, gonadotropin dose and E2 levels are used as criteria for defining poor ovarian response (15). However, the criteria for defining poor responders may also vary according to the specialist. Many screening tests such as ovarian reserve, CC challenge, GnRH, GnRH agonist, measurement of anti-Mullerian hormone (AMH) and antral follicular count (AFC) have been introduced overtime (15,17). Poor ovarian response occurred in 9-24% of all IVF cycles and is defined as decreased ovarian response with sufficient stimulation (18). Malmusi et al. described poor responders as patients with a low number of oocytes (less than 4), and no ovarian response with FSH greater than 300 IU (19). Poor response has been shown to be associated with advanced maternal age that affects oocyte quality and follicle numbers. This has also been observed in some young patients, but the causes are unclear (15,17). Although many studies are conducted to identify which protocol is suitable for patients in each of the response category, there is no definite consensus on the matter since each protocol comes with both benefits and limitations.
GnRH agonist long protocol versus GnRH antagonist protocol
The main side effects of GnRH agonist long protocol include longer treatment duration, more ampoules of gonadotropin, ovarian cyst formation, and menopausal syndromes (e.g., hot flushes, vaginal dryness etc). However, the antagonist protocol can overcome these side effects, but its disadvantage is low follicular production (20). Furthermore, the antagonist protocol has lower pregnancy and implantation rate because of low LH level and impaired estrogen secretion (21). Compared to patients treated with the antagonist protocol, patients treated with the agonist protocol demonstrated a significantly higher number of oocyte retrieved and mature oocytes production (with P value <0.05), while the cycle cancellation rate was similar (7,19,22). High numbers of oocytes produced with the agonist long protocol suggested that the protocol also improved the number of embryos produced. Therefore, GnRH agonist long protocol may be beneficial with regard to high cumulative pregnancy rate. A study conducted by Rabinson et al. also favored the agonist protocol in patients with normal body mass index (BMI) and showed no difference in the efficacy between the two protocols in patients with high BMI (>40); Therefore, BMI may be an important factor in deciding the starting dose of gonadotropin and the treatment protocol (23). High dose gonadotropin was required in patients with a high BMI to produce good ovarian stimulation (23). These studies accounted for patients representing all response types including those with poor response and polycystic ovary syndrome (PCOS).
Controversially, another study in the same year concluded that the antagonist protocol produced significantly high oocyte numbers (P=0.022) in poor responders who were previously subjected to GnRH long protocol treatment (24). Some studies have implicated the antagonist protocol in the prevention of moderate or severe ovarian hyperstimulation syndrome (OHSS), especially in women with PCOS, because of its rapid suppression of gonadotropin (i.e., a shorter cycle length, low estrogen level on day of HCG administration and a lower number of oocytes than agonist) (25,26). However, another similar study advised against the conclusion that the antagonist protocol is more efficacious compared to the agonist long protocol in terms of OHSS prevention due to lack of larger randomized trials with sufficient sample size and standard definitions of OHSS grades. This study showed no difference in the overall OHSS prevention between the two protocols; the difference was limited to severe OHSS (27). In fact, the study by Alama et al. found administration of a GnRH agonist following HCG administration to be an important strategy to prevent OHSS (28). The use of GnRH agonist as a final trigger in oocyte maturation is considered to minimize the risk of OHSS. However its use as a trigger wasn’t fully understood until the development of the GnRH antagonist. Even though the antagonist protocol is considered to be better at preventing OHSS, the risk still persists when HCG is used for the final maturation. This causes increased vascular endothelial growth factor expression in granulosa cells leading to increased vascular permeability and fluid shifting (28). This can be prevented by using the GnRH agonist for the final triggering of oocyte maturation. In addition, the use of GnRH agonist can decrease expression of vascular endothelial growth factor, inhibin B and steroidogenesis gene leading to minimal or no risk of OHSS (29).
On a side note, the GnRH antagonist protocol showed added risk of major congenital malformation such as Beckwith-Wiedemann syndrome and minor malformations such as naevus, skin tags, torticollis, pyloric stenosis and asymmetric head shape (30). Overall, the GnRH agonist long protocol showed better fulfillment in the purpose of controlled ovarian stimulation, which is to attain a greater number of mature follicles, with comparatively lower risks compared to the antagonist protocol.
Effectiveness of the minimal stimulation protocol
The antiestrogenic effects of CC suppress the premature LH surge while maintaining a positive influence on follicular development. The minimal stimulation protocol is a convenient protocol, which uses significantly fewer gonadotropin ampoules. The number of gonadotropin ampoules used in this protocol is significantly lower than agonist (5.7 vs. 25) (1). This protocol has resulted in less mature oocytes; consequently, lower chance of obtaining viable frozen embryos. However, the pregnancy and transplantation rate appeared to be similar with the agonist protocol (4,31). This protocol is cost-effective for women with advance age or for those with poor ovarian reserve compared to agonist or antagonist protocols. Additional studies have yielded a similar result when comparing the minimal stimulation protocol to GnRH agonist (i.e., CC and gonadotropin protocol was not as effective as agonist in yielding more oocytes but the transplantation and pregnancy rate were comparable between these protocols) (32,33). This protocol seemed to be a better option in some patients, such as those with poor ovarian response, when considering its cost-effectiveness and low risk of OHSS (34).
Some other limitations of using gonadotropins and CC in IVF included the higher prospect of multiple pregnancies, which was associated with preterm delivery, growth retardation and miscarriage. Although the correlation between ovarian stimulation and low birth weight is still debatable since it could be the confounding effect of the infertility background of the couple (35). Exposure of oocytes to the high levels of gonadotropins in their developing phase leads to improper maturation of oocyte as well as incomplete meiotic division which results in chromosomal aneuploidy (36). A study in a mouse model showed an increased rate of chromosomal aberrations in the female pronucleus in zygotes formed by ovarian stimulation (25). A similar study has also found an increased rate of aneuploidy in the chromosomes and mosaicism in an in vitro fertilized embryo (37,38). Baart et al. also concluded that the high dose FSH protocol caused a higher rate of mitotic segregation errors leading to mosaicism and hence abnormal embryos compared to the minimal stimulation protocol with low dose FSH (38). Moreover, congenital malformations like ventricular septal defect, cardiac defects and chromosomal abnormalities were found in patients undergoing IVF using CC (30).
Letrozole is also effective for ovulation induction in clomiphene resistant cases. It is considered superior to clomiphene because (I) it maintains endometrial thickness as it does not deplete estrogen receptors throughout the body; (II) it doesn’t hamper the negative feedback mechanism of the hypothalamo-pituitary-ovarian axis, and thus allows mono-follicular growth decreasing chances of multiple pregnancies (39). Studies have also shown that letrozole has a better ovulation and pregnancy rates than clomiphene in patients with PCOS. However, the use of the drug has been associated with congenital anomalies and has not been approved for use in ovulation induction (40,41).
Overall, minimal stimulation is an attractive option for many physicians and patients because of its lower cost; nevertheless, it is associated with higher risk factors compared to the other two protocols.
Effect of stimulation protocols on prenatal outcome
Overall, ovarian stimulation disturbs the methylation of differentially methylated regions (DMRs) causing imprinting defects. Studies have found that genes, which are imprinted in the later stage of developing oocytes, were affected the most. Imprinted genes are essential for the growth and development of embryos as well as placental function (37,42,43). DNA methylation is one of the important mechanisms for controlling gene imprints, hence methylation defects are most likely responsible for number of genetic diseases such as Angelman syndrome (defect at DMRs of SNRPN), Silver-Russell syndrome (defect in PEG1/MEST) and Beckwith-Wiedemann syndrome (defect of methylation in DMRs of KCNQ1OT1). Such imprinting defects have been identified in children conceived with assisted reproductive technologies (43).
Summary
This review summarizes the available evidence from previously published literatures regarding the efficacy of three different IVF protocols: (I) GnRH agonist long protocol; (II) GnRH antagonist protocol; and (III) minimal stimulation protocol. This review comprehensively examines patients in all response criteria, and confirms that all protocols come with a certain cost benefit ratio and has an overall jeopardy of epigenetic abnormalities. A close assessment of cost benefit factors demonstrates that the agonist long protocol is a better option for IVF stimulation compared to the antagonist and minimal stimulation protocols.
The main goal of COH is to obtain a greater numbers of mature follicles by suppressing the premature LH surge. GnRH agonist long protocol achieves a higher number of mature follicles compared to the other protocols. The use of high doses of gonadotropin and longer duration of treatment in this protocol has made many physicians to opt for other options such the antagonist and/or minimal stimulation protocols. However, GnRH agonist long protocol undoubtedly offers advantages of higher number of oocytes and viable embryos, which can be cryopreserved and used for frozen embryo transfer with better outcomes in patients with extreme BMIs and advanced age.
The minimal stimulation protocol using CC and gonadotropin is an attractive option considering its lower cost and gonadotropin ampoules as well as similar pregnancy and transplantation rates to GnRH agonist protocol. This protocol also limits the daily monitoring visits and ultrasonography compared to the standard protocol; hence this protocol can be considered a better option in patients with poor ovarian reserve and poor responders. Nevertheless, this protocol has the major disadvantage of decreased follicular development for patients with normal ovarian function.
This review highly demands additional studies conducted in a larger scale, with higher patient numbers and better standards of response definition and screening criteria, to achieve a more cogent conclusion. In the future it is also crucial to compare of these protocols with regard to the epigenetic aspects of prenatal outcomes.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Williams SC, Gibbons WE, Muasher SJ, et al. Minimal ovarian hyperstimulation for in vitro fertilization using sequential clomiphene citrate and gonadotropin with or without the addition of a gonadotropin-releasing hormone antagonist. Fertil Steril 2002;78:1068-72. [PubMed]
- Itskovitz-Eldor J, Kol S, Mannaerts B. Use of a single bolus of GnRH agonist triptorelin to trigger ovulation after GnRH antagonist ganirelix treatment in women undergoing ovarian stimulation for assisted reproduction, with special reference to the prevention of ovarian hyperstimulation syndrome: preliminary report: short communication. Hum Reprod 2000;15:1965-8. [PubMed]
- Zhang J, Chang L, Sone Y, et al. Minimal ovarian stimulation (mini-IVF) for IVF utilizing vitrification and cryopreserved embryo transfer. Reprod Biomed Online 2010;21:485-95. [PubMed]
- Ibrahim AE. The Minimal Stimulation Protocol for ICSI: An Alternative Protocol for Ovarian Stimulation. N Y Sci J 2014;7:19-23.
- Marci R, Caserta D, Lisi F, et al. In vitro fertilization stimulation protocol for normal responder patients. Gynecol Endocrinol 2013;29:109-12. [PubMed]
- Mohsen IA, El Din RE. Minimal stimulation protocol using letrozole versus microdose flare up GnRH agonist protocol in women with poor ovarian response undergoing ICSI. Gynecol Endocrinol 2013;29:105-8. [PubMed]
- Franco JG Jr, Baruffi RL, Mauri AL, et al. GnRH agonist versus GnRH antagonist in poor ovarian responders: a meta-analysis. Reprod Biomed Online 2006;13:618-27. [PubMed]
- Daya S. Gonadotropin releasing hormone agonist protocols for pituitary desensitization in in vitro fertilization and gamete intrafallopian transfer cycles. Cochrane Database Syst Rev 2000;CD001299. [PubMed]
- van Loenen AC, Huirne JA, Schats R, et al. GnRH agonists, antagonists, and assisted conception. Semin Reprod Med 2002;20:349-64. [PubMed]
- Grow D, Kawwass JF, Kulkarni AD, et al. GnRH agonist and GnRH antagonist protocols: comparison of outcomes among good-prognosis patients using national surveillance data. Reprod Biomed Online 2014;29:299-304. [PubMed]
- Kara M, Aydin T, Aran T, et al. Comparison of GnRH agonist and antagonist protocols in normoresponder patients who had IVF-ICSI. Arch Gynecol Obstet 2013;288:1413-6. [PubMed]
- Khalaf M, Mittre H, Levallet J, et al. GnRH agonist and GnRH antagonist protocols in ovarian stimulation: differential regulation pathway of aromatase expression in human granulosa cells. Reprod Biomed Online 2010;21:56-65. [PubMed]
- Lai Q, Zhang H, Zhu G, et al. Comparison of the GnRH agonist and antagonist protocol on the same patients in assisted reproduction during controlled ovarian stimulation cycles. Int J Clin Exp Pathol 2013;6:1903-10. [PubMed]
- Ziadeh SM, Zakaria MR, Abu-Hieja A. Pregnancy rates using CC/hMG or hMG alone. J Obstet Gynaecol Res 1997;23:97-101. [PubMed]
- Oehninger S. Poor responders in in vitro fertilization (IVF) therapy: the -challenge continues. Facts Views Vis Obgyn 2011;3:101-8. [PubMed]
- Davis OK. IVF stimulation: protocols for poor responders. Methods Mol Biol 2014;1154:329-41. [PubMed]
- Oudendijk JF, Yarde F, Eijkemans MJ, et al. The poor responder in IVF: is the prognosis always poor?: a systematic review. Hum Reprod Update 2012;18:1-11. [PubMed]
- Tarlatzis BC, Zepiridis L, Grimbizis G, et al. Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum Reprod Update 2003;9:61-76. [PubMed]
- Malmusi S, La Marca A, Giulini S, et al. Comparison of a gonadotropin-releasing hormone (GnRH) antagonist and GnRH agonist flare-up regimen in poor responders undergoing ovarian stimulation. Fertil Steril 2005;84:402-6. [PubMed]
- Kim CH, You RM, Kang HJ, et al. GnRH antagonist multiple dose protocol with oral contraceptive pill pretreatment in poor responders undergoing IVF/ICSI. Clin Exp Reprod Med 2011;38:228-33. [PubMed]
- A double-blind, randomized, dose-finding study to assess the efficacy of the gonadotrophin-releasing hormone antagonist ganirelix (Org 37462) to prevent premature luteinizing hormone surges in women undergoing ovarian stimulation with recombinant follicle stimulating hormone (Puregon). The ganirelix dose-finding study group. Hum Reprod 1998;13:3023-31. [PubMed]
- Xiao J, Chang S, Chen S. The effectiveness of gonadotropin-releasing hormone antagonist in poor ovarian responders undergoing in vitro fertilization: a systematic review and meta-analysis. Fertil Steril 2013;100:1594-601.e1-9.
- Rabinson J, Meltcer S, Zohav E, et al. GnRH agonist versus GnRH antagonist in ovarian stimulation: the influence of body mass index on in vitro fertilization outcome. Fertil Steril 2008;89:472-4. [PubMed]
- Marci R, Caserta D, Dolo V, et al. GnRH antagonist in IVF poor-responder patients: results of a randomized trial. Reprod Biomed Online 2005;11:189-93. [PubMed]
- Al-Inany HG, Youssef MA, Aboulghar M, et al. GnRH antagonists are safer than agonists: an update of a Cochrane review. Hum Reprod Update 2011;17:435. [PubMed]
- Lainas TG, Sfontouris IA, Zorzovilis IZ, et al. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF: a prospective randomised controlled trial (RCT). Hum Reprod 2010;25:683-9. [PubMed]
- Pundir J, Sunkara SK, El-Toukhy T, et al. Meta-analysis of GnRH antagonist protocols: do they reduce the risk of OHSS in PCOS? Reprod Biomed Online 2012;24:6-22. [PubMed]
- Alama P, Bellver J, Vidal C, et al. GnRH analogues in the prevention of ovarian hyperstimulation syndrome. Int J Endocrinol Metab 2013;11:107-16. [PubMed]
- Haas J, Ophir L, Barzilay E, et al. GnRH agonist vs. hCG for triggering of ovulation--differential effects on gene expression in human granulosa cells. PLoS One 2014;9:e90359. [PubMed]
- Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril 2006;85:1761-5. [PubMed]
- D'Amato G, Caroppo E, Pasquadibisceglie A, et al. A novel protocol of ovulation induction with delayed gonadotropin-releasing hormone antagonist administration combined with high-dose recombinant follicle-stimulating hormone and clomiphene citrate for poor responders and women over 35 years. Fertil Steril 2004;81:1572-7. [PubMed]
- Weigert M, Krischker U, Pöhl M, et al. Comparison of stimulation with clomiphene citrate in combination with recombinant follicle-stimulating hormone and recombinant luteinizing hormone to stimulation with a gonadotropin-releasing hormone agonist protocol: a prospective, randomized study. Fertil Steril 2002;78:34-9. [PubMed]
- Hwang JL, Huang LW, Hsieh BC, et al. Ovarian stimulation by clomiphene citrate and hMG in combination with cetrorelix acetate for ICSI cycles. Hum Reprod 2003;18:45-9. [PubMed]
- Albuquerque LE, Tso LO, Saconato H, et al. Depot versus daily administration of gonadotrophin-releasing hormone agonist protocols for pituitary down regulation in assisted reproduction cycles. Cochrane Database Syst Rev 2013;1:CD002808. [PubMed]
- Kapiteijn K, de Bruijn CS, de Boer E, et al. Does subfertility explain the risk of poor perinatal outcome after IVF and ovarian hyperstimulation? Hum Reprod 2006;21:3228-34. [PubMed]
- Hodges CA, Ilagan A, Jennings D, et al. Experimental evidence that changes in oocyte growth influence meiotic chromosome segregation. Hum Reprod 2002;17:1171-80. [PubMed]
- Katz-Jaffe MG, Trounson AO, Cram DS. Chromosome 21 mosaic human preimplantation embryos predominantly arise from diploid conceptions. Fertil Steril 2005;84:634-43. [PubMed]
- Baart EB, Martini E, Eijkemans MJ, et al. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod 2007;22:980-8. [PubMed]
- Kar S. Current evidence supporting "letrozole" for ovulation induction. J Hum Reprod Sci 2013;6:93-8. [PubMed]
- Begum MR, Ferdous J, Begum A, et al. Comparison of efficacy of aromatase inhibitor and clomiphene citrate in induction of ovulation in polycystic ovarian syndrome. Fertil Steril 2009;92:853-7. [PubMed]
- Biljan MM, Hemmings R, Brassard N. The Outcome of 150 Babies Following the Treatment With Letrozole or Letrozole and Gonadotropins. Fertility and sterility 2005;84:S95.
- Fortier AL, Lopes FL, Darricarrère N, et al. Superovulation alters the expression of imprinted genes in the midgestation mouse placenta. Hum Mol Genet 2008;17:1653-65. [PubMed]
- Lawrence LT, Moley KH. Epigenetics and assisted reproductive technologies: human imprinting syndromes. Semin Reprod Med 2008;26:143-52. [PubMed]


