Pulmonary arteriovenous malformation-etiology, clinical four case presentations and review of the literature
Introduction
Pulmonary arteriovenous malformation (PAVM) is a rare clinical condition with abnormal direct communication between the branches of pulmonary artery and vein. Also, it is known as pulmonary arteriovenous fistula, pulmonary arteriovenous aneurisms and pulmonary hemangioma. It was described for the first time in 1897 on autopsy of a young man (Churton). First PAVM described in a live patient was in 1939. From 1942 to 1977 surgery was known as the only therapy for the patients with PAVM and included ligature of PAVM, local excision, segmentectomy, lobectomy and even pneumonectomy. Liu et al. published in 2010, the first successful resection of PAVM by video-assisted thoracoscopy (1-3). Endovascular embolisation of PAVM is characterized by high efficacy and rare complications (1). The most serious complication is paradoxical embolisation, which usual happened in carotid artery, celiac artery, superior mesenteric or iliac artery, with incidence of 0.5-2.5% (4). Incidence of PAVM is about 1:50,000 cases (1), and less than 400 cases has been described in the literature, so it is very important to have in mind that clinical and radiological scenario can establish the right diagnosis of PAVMs.
Case reports
Patient No. 1
A 65-year-old female patient was admitted to hospital due to recurrent hemoptysis, arterial hypertension and recurrent epistaxis that persisted over a month. She denied previous thoracic trauma, bacterial or tuberculosis (TBC) infections or other hereditary diseases. Six months earlier, she was diagnosed as having arterial hypertension and took medications regularly. The chest CT revealed only consolidation of the right lower lobe. No pathologically enlarged mediastinal lymph nodes or other nodes were seen. Pulmonary thromboembolism has been excluded. On bronchoscopy bleeding has been found from the bronchus for the right lower lobe. After the next episode of hemoptysis, the right anterolateral thoracotomy and right low lobectomy has been performed. Her postoperative course was uneventful, without further hemoptysis, postoperative bronchoscopy was performed and the results were normal.
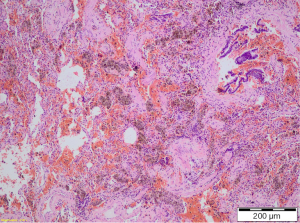
The light microscopy examination showed subcentimeter PAVM beside the resected bronchus for the lower lobe (Figure 1). The patient was discharged from the hospital and a year later she is without recurrence of hemoptysis (Figure 1).
Patient No. 2
A 74-year-old male patient was admitted to hospital due to recurrent hemoptysis which lasts for 14 days. He denied previous respiratory or cardiovascular diseases, thoracic trauma nor infection. He is smoker two packs per day for 50 years. The chest CT revealed several consolidations in right lower lobe. On bronchoscopy active bleeding has been found from the bronchus for the right upper lobe, with some clots in intermedius bronchus and bronchus for the right lower lobe. After the next episode of hemoptysis, the right anterolateral thoracotomy and right upper lobectomy has been performed. Postoperative course was uneventful, without further hemoptysis, postoperative bronchoscopy was performed, the results were normal and patient was discharged from the hospital and a year later she is without recurrence of hemoptysis.
Patient No. 3
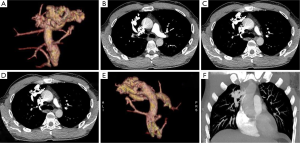
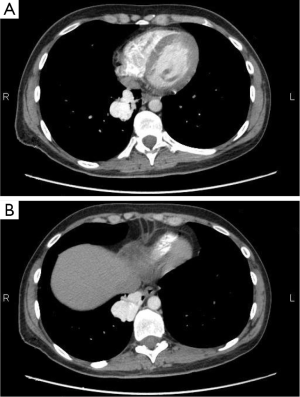
A 39-year-old male patient was admitted to hospital due to radiological finding of PAVM in right upper lobe, found in asymptomatic state. The chest CT revealed in right upper lobe PAVM diameter of 5 cm. Feeding artery was the branch of the right pulmonary artery and drainage veins were branches of right upper pulmonary vein. There were no other pathologic findings in lungs or mediastinum, beside bullous emphysema (Figure 2A-C). Patient denied previous respiratory or cardiovascular diseases. A right anterolateral thoracotomy was performed; dissection of PAVM vessels and atypical (wedge) resection of whole PAVM was performed with TA staplers. Postoperative course was uneventful and patient was discharged from the hospital after 7 days (Figure 2).
Patient No. 4
A 33-year-old female patient was admitted to hospital due to radiological finding of PAVM in right lower lobe, found in asymptomatic state. The chest CT revealed in right lower lobe PAVM diameter of 4.5 cm. Feeding artery was the branch of the right lower lobar artery and drainage veins were branches of right lower pulmonary vein. There were no other pathologic findings in lungs or mediastinum, beside bullous emphysema (Figure 2A). Patient denied previous respiratory or cardiovascular diseases. A right anterolateral thoracotomy was performed; dissection of PAVM vessels and atypical (wedge) resection of whole PAVM was performed with TA staplers. Postoperative course was uneventful and patient was discharged from the hospital after 4 days (Figure 3).
Discussion
PAVM is characterized by direct communication of pulmonary artery and pulmonary vein, without intervening capillary. Incidence of PAVM is about 1:50,000 cases (1), and less than 400 cases has been described in the literature. It occurs twice as often in women as in men, but there is a male predominant in newborns (3-6). In 53-70% cases has been found in lower lobes, in 75% is unilateral, 36% multiple, and half of multiple lesions has bilateral localization. In 81% cases localization is subpleural (3).
PAVM can be simple or complex, then primary or secondary, large or small. Simple PAVM are characterized by direct communication between one pulmonary artery and one pulmonary vein, and recommendation therapy for this type of PAVM is transcatheter embolisation (7). Complex PAVM has two or more different segmental arteries supplying the aneurismal sac and one or two draining veins. Simple PAVM occurs in about 80% and complex in 20% (3). The golden standard for diagnosis of complex PAVM is pulmonary arteriography (2).
Primary or congenital PAVM are more often, and secondary or acquired are caused by infection and occur due to hyperplastic changes in bronchial arteries or abnormal communication between pulmonary artery and vein (tuberculosis, actinomycosis, schistosomiasis) metastatic thyroid carcinoma, thoracic trauma, hepatic cirrhosis, mitral stenosis (7,8) modified Fountain’s operation or iatrogenic (9,10).
Due to the size, PAVM can be divided as small (less than 1 to 5 cm) or big (more than 5 cm) and can fulfill the whole hemithorax.
In most cases, PAVM are asymptomatic until the four decade of patient’s life (11).
The most common clinical sign of PAVM is epistaxis. Dispnea is the second common clinical sign and is usually present in patients with big or multiple PAVM. Hemoptysis is the third most common sign, and after that are hypoxia, central cyanosis, clubbing fingers, hematothorax, and brain abscess. About 30-40% patients with PAVM will have cerebrovascular insult or transitory ischemic attack later in life (5).
One of the hallmarks of PAVM is orthodeoxia, which is characterized by decreased of oxygen saturation upon standing. It is caused by blood pooling at the lung base, where the most PAVM is located.
Diagnosis of PAVM is usually quite difficult, because the first sign can be massive hemoptysis or hematothorax, and the definitive diagnosis will be established on pathohistological examination after surgical intervention. The golden standard for achieving the diagnosis of PAVM today is pulmonary angiography, and the standard procedure today is helical CT scan (12). Recently, many studies suggest that contrast echocardiography with chest X-ray has 100% sensitivity, negative predictive value; it is cheaper and less invasive, less exposure to radiation and available in smaller medical centers (12-14).
Differential diagnosis bronchial PAVM to some other PAVM is characterized by predominantly male population, right lung localization, solitary lesion and without hereditary component or Rendu-Osler-Weber syndrome (15).
Hereditary hemorrhagic telangiectasia (HHT) is the most common vascular abnormality in lungs. The classic clinical presentation is epistaxis, dilated blood vessels over lips and fingers and gastrointestinal bleeding. The diagnostic criteria for HHT include three of the four following conditions: recurrent epistaxis, telangiectasia elsewhere from the nasal mucosa, autosomal dominant inheritance and visceral involvement (16-20). HHT is hereditary diseases with gene on the 9th chromosome q33-34. Patients with more than one PAVM have 90% chances to suffer from HHT (5,21,22). If the PAVM is located closer to visceral pleura, then hematothorax is more common clinical presentation, and if it is closer to bronchi, then hemoptysis are more often (15). Patients with PAVM are recommended to have antibiotics prophylaxis before any surgical or dental procedure (11).
There are two recommended therapeutic options in treatment of patients with PAVM: transcatheter embolisation and surgical resection (ligature, excision, segmentectomy, lobectomy, pneumonectomy). Transcatheter embolisation is less invasive technique in treatment of PAVM with success of 85-98% of cases. In cases of massive hemoptysis or hematothorax, surgery is the therapy of choice, which eradicates the PAVM, and after the transcatheter embolisation there is a chance of PAVM revascularization (15).
Surgical resection of PAVM is recommended for all the patients who can submit general anesthesia, if transcatheter embolisation failed, for patients with neurological complications, newborns or central localization of PAVM. In patients with non-diagnosed massive hemoptysis, surgical resection has small percentage of perioperative mortality 0-9% (22).
Since there are less than 400 cases of PAVM described in the literature, it is very important to have in mind that clinical and radiological scenario can establish the right diagnosis of PAVM and appropriate treatment of this condition.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Liu FY, Wang MQ, Fan QS, et al. Endovascular embolization of pulmonary arteriovenous malformations. Chin Med J (Engl) 2010;123:23-8. [PubMed]
- Chowdhury UK, Kothari SS, Bishnoi AK, et al. Successful lobectomy for pulmonary arteriovenous malformation causing recurrent massive haemoptysis. Heart Lung Circ 2009;18:135-9. [PubMed]
- Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med 1998;158:643-61. [PubMed]
- Díaz-Aguilera R, Zurera-Tendero LJ, Canis-López M, et al. Embolotherapy of pulmonary arteriovenous malformations: long-term clinical and radiological follow-up. Radiologia 2009;51:85-9. [PubMed]
- Borrero CG, Zajko AB. Pulmonary Arteriovenous malformations: clinical features, diagnosis, and treatment. J Radiol Nurs 2006;25:33-7.
- Cottin V, Plauchu H, Bayle JY, et al. Pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. Am J Respir Crit Care Med 2004;169:994-1000. [PubMed]
- Pelage J, Lagrange C, Chinet T, et al. Embolization of localized pulmonary arteriovenous malformations in adults. J Radiol 2007;88:367-76. [PubMed]
- Wong HH, Chan RP, Klatt R, et al. Idiopathic pulmonary arteriovenous malformations: clinical and imaging characteristics. Eur Respir J 2011;38:368-75. [PubMed]
- Faughnan ME, Granton JT, Young LH. The pulmonary vascular complications of hereditary haemorrhagic telangiectasia. Eur Respir J 2009;33:1186-94. [PubMed]
- Khurshid I, Downie GH. Pulmonary arteriovenous malformation. Postgrad Med J 2002;78:191-7. [PubMed]
- Kessler CS, Leipzig SM. A 24-year-old man with chest pain, hemoptysis, and hypoxia. Am J Emerg Med 2008;26:904-7. [PubMed]
- Liechty KW, Flake AW. Pulmonary vascular malformations. Semin Pediatr Surg 2008;17:9-16. [PubMed]
- van Gent MW, Post MC, Luermans JG, et al. Screening for pulmonary arteriovenous malformations using transthoracic contrast echocardiography: a prospective study. Eur Respir J 2009;33:85-91. [PubMed]
- Parra JA, Bueno J, Zarauza J, et al. Graded contrast echocardiography in pulmonary arteriovenous malformations. Eur Respir J 2010;35:1279-85. [PubMed]
- Miyoshi K, Moriyama S, Nawa S. Bronchial arteriovenous malformation with large aneurysm, resected by video-assisted thoracic surgery. Gen Thorac Cardiovasc Surg 2009;57:162-5. [PubMed]
- Supakul N, Fan R, Karmazyn B. A case report: Pulmonary venous malformation complicated with pulmonary hemorrhage. J Pediatr Surg 2012;47:e35-8. [PubMed]
- Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet 2011;48:73-87. [PubMed]
- Litzler PY, Douvrin F, Bouchart F, et al. Combined endovascular and video-assisted thoracoscopic procedure for treatment of a ruptured pulmonary arteriovenous fistula: Case report and review of the literature. J Thorac Cardiovasc Surg 2003;126:1204-7. [PubMed]
- Bandyopadhyay SK, Nandy A, Sarkar S, et al. Massive haemothorax: a presentation of pulmonary arteriovenous malformation. Indian J Chest Dis Allied Sci 2008;50:285-7. [PubMed]
- Heimdal K, Dalhus B, Rødningen OK, et al. Mutation analysis in Norwegian families with hereditary hemorrhagic telangiectasia: founder mutations in ACVRL1. Clin Genet 2015. [Epub ahead of print]. [PubMed]
- Heald B, Rigelsky C, Moran R, et al. Prevalence of thoracic aortopathy in patients with juvenile polyposis syndrome-hereditary hemorrhagic telangiectasia due to SMAD4. Am J Med Genet A 2015. [Epub ahead of print]. [PubMed]
- Georghiou GP, Berman M, Vidne BA, et al. Pulmonary arteriovenous malformation treated by lobectomy. Eur J Cardiothorac Surg 2003;24:328-30. [PubMed]