Management of diabetic renal disease
Introduction
Diabetic nephropathy comprises a triad of albuminuria, hypertension and declining renal function. It is defined by the presence of microalbuminuria in a person with diabetes and this detection heralds the onset of a dramatically increased risk of death, frequently as a result of premature cardiovascular disease (1). Furthermore, other microvascular complications such as proliferative retinopathy and neuropathy tend to occur more frequently in this group of patients.
This article aims to review our knowledge of the natural history of this condition, and the potentials for screening and early therapy to prevent progression of this most devastating complication of diabetes.
Epidemiology
Early studies suggest that nephropathy affects around 30% of patients with type 1 diabetes and 20% of patients with type 2 diabetes (2). Duration of diabetes is a major risk factor for the development of nephropathy, so point prevalence studies are difficult to interpret. More recent studies, however, suggest a decline in the incidence of the condition, particularly in patients with type 1 diabetes. Men appear to be at a higher risk of developing nephropathy, and ethnicity appears to be an important risk factor, with South-Asians and African-Caribbeans being at higher risk (3,4).
In the UK, it is estimated that around one third of patients with end stage renal failure (ESRF) develop the condition due to diabetes (5). Patients with nephropathy have an increase in relative mortality of 40-100 times that of non-diabetic subjects, and the excess cardiovascular mortality in patients with diabetes is mainly confined to this group of patients. Early studies show a 5-year survival after the onset of persistent albuminuria of 65%, although the corresponding figure at 10 years is only 28% (6). More recent statistics suggest a dramatically improved prognosis in diabetic nephropathy to around an 82% 10-year survival, predominantly due to advances in blood pressure therapy and renal replacement therapy.
Natural history
The natural history of nephropathy has been well characterised in type 1 diabetes, but less well so in type 2 diabetes. The earliest detectable physiological change associated with progression to nephropathy is renal enlargement mostly due to tubular hypertrophy and hyperplasia in response to glycosuria. These changes are not completely reversible with glycaemic control, and their link to later nephropathy is uncertain. Glomerular filtration rate (GFR) is also increased in newly diagnosed patients (termed glomerular hyperfiltration) but this usually returns to normal with glycaemic correction. Some studies have suggested an association between higher GFR and subsequent progression to nephropathy—the presence or absence of glomerular hyperfiltration has a positive predictive value for progression to microalbuminuria or overt nephropathy of 53%, and a negative predictive value of >95%, respectively (7). More recent long-term prospective surveys, however, suggest that glomerular hyperfiltration does not play a major role in the prediction of renal outcome in type 1 diabetes (8).
The raised GFR in people with newly diagnosed type 1 diabetes (and to a lesser extent type 2) has been shown in experimental studies to be due to dilatation of the afferent glomerular arteriole. This leads to an increase in glomerular capillary pressure which drives increased filtration (hyperfiltration). The increased pressure results in mechanical stress in the GBM which, in turn, is thought to stimulate matrix production and thickening via production of pro-fibrotic cytokines, such as transforming growth factor-β (TGF-β) (9). Angiotensin II is thought to be a key mediator of these changes. Hyperfiltration has been associated with later nephropathy development in type 1 diabetes, but the link was no longer significant when corrected for hyperglycaemia. Its role in type 2 diabetes is much less certain.
Incipient nephropathy is characterised by the presence of microalbuminuria [albumin excretion rate (AER)] 20-200 mcg/min or 30-300 mg/24 h), and unchecked may lead to overt nephropathy with macroalbuminuria (AER >200 mcg/min or >300 mg/24 h). Once persistent albuminuria develops, AER increases by approximately 20% per year if left untreated. The GFR starts to fall when AER reaches around 100 mcg/min, declining at a rate of around 10 mL/min/1.73 m2 per year if untreated. Serum creatinine begins to rise when GFR is less than 50 mL/min/1.73 m2, and an inexorable decline into renal failure ensues.
Microalbuminuria
Type 1 diabetes
The prevalence of microalbuminuria in type 1 diabetes is around 20%. Two reports in the early 1980’s suggested that the presence of microalbuminuria in patients with type 1 diabetes was highly predictive of progression to overt nephropathy, with around 80% of patients with microalbuminuria progressing to overt nephropathy (10,11). It is of note, however, that the total number of microalbuminuric patients studied in these two reports was only 22. In an inception cohort of 277 type 1 patients from Denmark, studied from 1979 to 2004, the cumulative incidence of microalbuminuria was 34% and for clinical nephropathy 15% after 20 years. More recent studies have shown less dramatic results. For End stage renal disease rates of 2.2% and 7.8% have recently been reported from Finland after 20 and 30 years duration respectively (12).
The presence of microalbuminuria does appear to be a potent risk marker for vascular disease in patients with type 1 diabetes. In a cohort study of 259 patients with type 1 diabetes, elevated AER was highly predictive of atherosclerotic vascular disease [hazard ratio, 1.06 (95% CI, 1.02-1.18) per 5 mg increase in AER], independent of other cardiovascular risk factors (13). Patients with microalbuminuria also appear to be at greater risk of silent atherogenic vascular disease (14).
Patients with type 1 diabetes and microalbuminuria appear to have features of the metabolic syndrome, which may contribute to their high level of cardiovascular morbidity, in particular dyslipidaemia (15). The co-existence of insulin resistance in people with type 1 diabetes has been termed Double Diabetes. It has been demonstrated that these patients have greater insulin resistance compared to their normoalbuminuria counterparts, and this may provide a link between microalbuminuria, hypertension, dyslipidaemia and type 1 diabetes (16).
Type 2 diabetes
In contrast to type 1 diabetes, the presence of microalbuminuria in patients with type 2 diabetes is not such a strong risk marker for progressive renal disease. Microalbuminuria is less common in patients with type 2 diabetes (around 14%), and commonly may revert to normoalbuminuria over prolonged follow up (17). In the short term, it has been observed that patients with microalbuminuria have a greater decline in GFR compared to normoalbuminuric type 2 diabetic subjects, although this decline does not always become clinically relevant. Renal biopsy studies of microalbuminuric type 2 diabetic patients have shown that a significant number of patients may have renal diagnoses other than diabetic glomerulopathy, suggesting that microalbuminuria may not strongly predict the presence of diabetic nephropathy (18).
Microalbuminuria does appear to be a very strong risk marker for vascular disease in patients with type 2 diabetes (19). In a systematic overview of eight cohort studies, a total of 2,138 type 2 diabetic patients were followed for a mean of 6.4 years (20). In the presence of microalbuminuria, an overall odds ratio for death was 2.4 (95% CI, 1.8-3.1), and cardiovascular death was 2.0 (95% CI, 1.4-2.7). Microalbuminuria is also a strong risk marker for vascular disease in non-diabetic populations (21). Similar findings to type 1 diabetes have been seen in patients with type 2 diabetes with respect to the metabolic syndrome and cardiovascular risk factors—patients with microalbuminuria appear to have greater insulin resistance, dyslipidaemia and hypertension compared to their normoalbuminuric counterparts (22), and hence the cardiovascular risk seen in these patients may be modulated by insulin resistance. Microalbuminuria has also been associated with insulin resistance in non-diabetic patients (23).
Screening for microalbuminuria
A number of consensus statements have suggested that screening for microalbuminuria in type 1 and type 2 diabetes is worthwhile in order to prevent progression to renal failure (1,24). This conclusion relies on the fact that anti-hypertensive therapy in microalbuminuric patients may attenuate progression, even in normotensive individuals (25). Cost benefit analyses for microalbuminuria screening in type 1 diabetes suggests that assuming a treatment benefit of 10%, screening would be economically neutral, and any benefit over this would lead to increased life expectancy and reduced ESRF (26). In type 2 diabetes, the argument for screening for microalbuminuria is less strong, but nevertheless widely acknowledged as beneficial, and recommended by many international guidelines.
Screening for microalbuminuria has the fundamental problem that intra-individual variability of AER is very wide, and varies according to diet, exercise and temperature (27). Timed urine collections are too cumbersome for routine clinical care, so spot samples (preferably first morning void samples) are used and in order to allow for urine concentration, the albumin content is corrected for creatinine giving an albumin:creatinine ratio (ACR). A positive ACR on two or more occasions is enough to confirm the diagnosis of diabetic renal disease (ACR >2.5 in males and >3.5 in females). This is suggested as giving a sensitivity of 96% and a specificity of 99.7% for the presence of diabetic nephropathy (28).
Historically, GFR declined at a rate of 10 mL/min/year once patients developed overt nephropathy, but this rate is now around 2-4 mL/min/year with effective blood pressure control. Rates of decline greater than this should prompt careful clinical review. The MDRD GFR equation (estimates GFR based on creatinine, age, sex and ethnicity—www.renal.org/egfrcalc) is not very precise at GFR levels >90 mL/min/1.73 m2, so it is difficult to detect changes in early diabetes prior to the development of albuminuria. Older people with type 2 diabetes may have chronic kidney disease (CKD) due to other non-diabetic causes, such as hypertension or renovascular disease. They may have a more benign course, with stable renal function for many years.
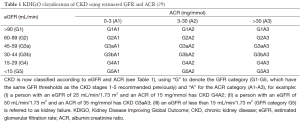
Classification of nephropathy is now based on the new Kidney Disease Improving Global Outcome (KDIGO) guidelines combination of estimated GFR (eGFR) and ACR (Table 1) (29).
Management of diabetic nephropathy
Monitoring of patients with diabetes and CKD
Patients with established diabetic nephropathy require careful monitoring for progression of renal disease, and other complications. All patients with diabetes should have yearly measurements of creatinine, eGFR, ACR and potassium. At an eGFR of 45-60, nephrology referral should be considered for non-diabetic renal disease or rapid fall in eGFR. At eGFR 30-44, more frequent monitoring of eGFR is required, along with monitoring haemoglobin and vitamin D status. Patients with eGFR <30 should be referred to a nephrologist for consideration/preparation for renal replacement therapy.
Many centres undertake care of patients with diabetic nephropathy in combined diabetic-renal clinics, run jointly by a diabetes specialists and nephrologists. This arrangement has the advantage of ease of referral between the specialties, and the clearly stated objective of reducing rate of decline of renal function once nephropathy is diagnosed. If renal function is declining to ESRF, the patient can be physically and psychologically prepared for replacement therapy. Furthermore, careful surveillance for retinopathy and neuropathy can be undertaken. The diabetic renal clinic setting has been audited and found to be of major benefit to patients approaching ESRF by postponing the need for dialysis by a mean of 2 years (5).
Anti-hypertensive treatment in diabetic nephropathy
Systemic hypertension is associated with diabetic nephropathy and indeed plays a role in its development (30). Those patients with persistent hypertension have a faster decline in renal function than those without (31). Vigorous anti-hypertensive therapy in patients with diabetic nephropathy can substantially retard progression of the disease. Early studies involved small numbers of patients with nephropathy but the results were highly significant showing a reduction in the annual rate of decline of GFR and also a reduction in AER (32,33). These studies used drugs such as beta-blockers, alpha-blockers, hydralazine, methyldopa and diuretics, reducing the rate of decline of GFR by approaching 50%. A drawback of these studies was the lack of control groups and small numbers, although their results have been reproduced in many subsequent studies.
The fact that ACE inhibitors have a beneficial effect in diabetic nephropathy is well established, with a reduction in albuminuria and rate of decline of GFR often observed. The mechanism underlying this is thought to be due to selective vasodilatation of efferent arterioles leading to a reduction of intraglomerular hypertension, enhancement of membrane selectivity and improvement in growth characteristics of glomerular tissue (34).
Meta-analysis using a random effects model showed that angiotensin converting enzyme inhibitors (ACEI) were more effective than calcium antagonists or placebo for preventing onset of microalbuminuria. ACEI and β blockers did not differ for onset of microalbuminuria (1 RCT, n=299; relative risk 1.01; 95% CI, 0.74-1.37) in diabetic patients without nephropathy. In diabetic patients with nephropathy, ACE inhibitors reduced all causes mortality more than placebo, but angiotensin receptor blockers (ARBs) did not (35). Both ACEI and ARBs reduced progression from micro- to macroalbuminuria, and ARBs reduced risk of ESRF and doubling of creatinine more than placebo.
Dual blockade of the renin angiotensin system (RAS)
This is rarely indicated and several studies lay testament to this. The Ontarget Trial used ramipril and telmisartan in patients with nephropathy, and the combination of the two drugs was associated with more adverse events without an increase in benefit (36). A similar study added aliskiren (a direct renin antagonist) to an ACEI or ARB (37). The independent safety committee recommended that the trial end early due to the increased adverse events (hyperkalemia and hypotension) and increased risk of stroke. The study showed that there was no benefit in cardiovascular and renal outcomes after adding aliskiren to current drug therapy. The outcome of this study was surprising, as other drugs that inhibit the RAS decrease the incidence of strokes and cardiovascular deaths. A further study, Olmesartan Reducing Incidence of Endstage Renal Disease in Diabetic Nephropathy Trial (ORIENT) examined the use of olmesartan to ACEI therapy in a Japanese and Chinese population (38). The study concluded that in patients with type 2 diabetes with overt nephropathy and renal insufficiency there was no further improvement in renal or cardiovascular outcomes.
Microalbuminuria and hypertension
Many studies suggest that anti-hypertensive therapy to microalbuminuric diabetic patients with hypertension can reduce AER and rate of decline of GFR (39). Again ACEI drugs have been found to have a specific beneficial effect above other anti-hypertensive therapies. In type 2 diabetes, treatment of microalbuminuria with ACEI can reduce AER and rate of decline of GFR significantly more than calcium antagonists (40).
Microalbuminuria and normotension
The question arises as to whether anti-hypertensive therapy in normotensive microalbuminuric subjects delays or prevents the onset of established nephropathy. Although microalbuminuric subjects commonly have normotension, they have blunting of diurnal blood pressure variation and slightly elevated blood pressure compared to age matched controls (41). As these patients often progress to develop hypertension, early therapy to achieve a modest reduction in blood pressure may prevent the onset of overt nephropathy. Mathiesen and colleagues conducted an open randomised study comparing captopril to placebo in 44 normotensive microalbuminuric type 1 diabetic subjects (42). They showed a significant reduction in AER in the treated group from 82 to 57 mg/24 h compared to the untreated group in whom an increase was seen from 105 to 166 mg/24 h. A large study by the European Microalbuminuria Captopril Study Group with 92 normotensive microalbuminuric type 1 diabetics has shown significantly less progression to clinical albuminuria and reduced albumin excretion in patients treated with captopril compared to placebo (43). The group concluded that captopril therapy even in normotensive patients with microalbuminuria retards the progression to overt nephropathy. This result is also confirmed in the Euclid study, examining anti-hypertensive therapy in normotensive type 1 diabetic patients with micro- and normoalbuminuria (25). A slight concern is that only 46% of patients with microalbuminuria progress to overt nephropathy and hence some patients may be treated unnecessarily. It is apparent, however, that ACEI drugs appear to have a beneficial role in renal preservation over other classes of drugs.
Lipid lowering therapy in diabetic nephropathy
There is experimental evidence to suggest that hypercholesterolaemia may play a pathogenic role in progressive glomerular injury. There is also evidence to suggest that reduction in cholesterol can reduce the rate of decline of GFR in patients with diabetic nephropathy. Lipid lowering therapy in rat models of hypertension can reduce AER, and in vitro can reduce mesangial expansion (44). Several small studies have shown a reduction in rate of decline of GFR and reduction in albuminuria in patients with overt diabetic nephropathy. Reduction of AER in normotensive, microalbuminuric type 2 diabetic patients has also been observed on statin therapy (45), and improved oxidative stress has also been noted (46). Furthermore, reduction in hypertriglyceridaemia using fibrate therapy can attenuate AER rise in patients with type 2 diabetes (47), although recent study has not confirmed this (48).
A further argument for the early use of lipid lowering therapy in patients with albuminuria or microalbuminuria is that as both are very strong cardiovascular risk markers, lipid lowering therapy should be instituted as a secondary prevention measure. In patients with established renal failure, statins rather than fibrates should be used.
Reduction in dietary protein
High dietary protein has been shown to damage the kidneys in experimental diabetes. In type 1 diabetes, protein restriction may reduce the rate of loss of GFR, albuminuria and mortality in people with established nephropathy. The data is less convincing in type 2 diabetes. In a recent meta-analysis of 13 RCTs enrolling 779 patients, a low-protein diet was associated with a significant improvement in GFR (5.82 mL/min/1.73 m2; 95% CI, 2.30-9.33; I2=92%; n=624) (49). This effect was consistent across the subgroups of type of diabetes, stages of nephropathy and intervention period.
Improvement in glycaemic control
Both the Diabetes Control and Complications Trial (DCCT) (50), and United Kingdom Prospective Diabetes Study (UKPDS) (51), confirmed that good glycaemic control can prevent the development of microalbuminuria. There are conflicting data on the role of glycaemic control in the progression of established nephropathy although as a rule, patients with worse control do less well and have associated complications such as retinopathy and neuropathy. As GFR declines, clearance by the kidneys is reduced, so doses of some hypoglycaemic therapies and insulin may need adjustment. There is also greater risk of hypoglycaemia and hypoglycaemia unawareness, so target glycated haemoglobin (HbA1c) may be higher than for those without diabetes.
Many national and international guidelines recommend individualised HbA1c targets (52). Two large studies investigating the role of improving glycaemic control to prevent the progression of diabetic nephropathy have been reported. The Microalbuminuria Collaborative Study Group examined 70 type 1 diabetic subjects with microalbuminuria, and randomised half to intensive insulin therapy and half to normal insulin therapy (53). No significant difference in progression of microalbuminuria to albuminuria was seen between the two groups. In the DCCT, although intensive glycaemic control reduced the onset of microalbuminuria and macroalbuminuria by 34% and 56% respectively, patients who had microalbuminuria at the commencement of the study did not have a reduction in progression to overt nephropathy with intensive glycaemic control (50).
Renal replacement therapy in patients with diabetes
Advances in renal replacement therapy mean that should ESRF occur as a result of diabetic nephropathy, acceptable therapies are available. The main concern is the high rate of cardiovascular death in patients on any form of dialysis, which is particularly true of diabetic subjects. Early transplantation is the treatment of choice, and use of the oxazoline derivative of prednisolone, deflazacort, as the main immunosuppressive drug may have the benefit of induction of less glucose intolerance and hence better diabetic control (54). Advances in combined renal and pancreatic transplantation can also result in amelioration of diabetes as well as renal replacement therapy.
Conclusions
Diabetic nephropathy is the most hazardous of the complications of diabetes, and is responsible for excess morbidity and mortality in patients with type 1 and type 2 diabetes. Advances in our understanding of the natural history of the condition have enabled us to intervene earlier in the disease process in order to modify the course of the disease. Prevention of nephropathy can be achieved by tight glycaemic and blood pressure control. Once microalbuminuria develops, ACEI drugs are the mainstay of therapy, along with cardiovascular risk reduction with statins. Patients with diabetic nephropathy require careful multi-disciplinary management to prevent or delay the onset of ESRF, and reduce the risk of other (particularly cardiovascular) complications.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 2014;37:2864-83. [PubMed]
- American Diabetes Association. Nephropathy in Diabetes. Diabetes Care 2004;27:579-83.
- Burden AC, McNally PG, Feehally J, et al. Increased incidence of endstage renal failure secondary to diabetes mellitus in Asian ethnic groups in the United Kingdom. Diabet Med 1992;9:641-5. [PubMed]
- Cowie CC, Port FK, Wolfe RA, et al. Disparities in incidence of diabetic end stage renal disease according to race and type of diabetes. N Engl J Med 1989;321:1074-9. [PubMed]
- Liew BS, Perry C, Boulton-Jones JM, et al. Diabetic nephropathy: an observational study on patients attending a joint diabetes renal clinic. QJM 1997;90:353-8. [PubMed]
- Parving HH, Hommel E. Prognosis in diabetic nephropathy. BMJ 1989;299:230-3. [PubMed]
- Rudberg S, Persson B, Dahlquist G. Increased glomerular filtration rate as a predictor of diabetic nephropathy--an 8-year prospective study. Kidney Int 1992;41:822-8. [PubMed]
- Premaratne E, Verma S, Ekinci EI, et al. The impact of hyperfiltration on the diabetic kidney. Diabetes Metab 2015;41:5-17. [PubMed]
- Kanwar YS, Wada J, Sun L, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008;233:4-11. [PubMed]
- Parving HH, Hommel E, Mathiesen E, et al. Prevalence of microalbuminuria, arterial hypertension, retinopathy and neuropathy in insulin dependent diabetic patients. Br Med J (Clin Res Ed) 1988;296:156-60. [PubMed]
- Viberti GC, Hill RD, Jarrett RJ, et al. Microalbuminuria as a predictor of clinical nephropathy in insulin dependent diabetes mellitus. Lancet 1982;1:1430-2. [PubMed]
- Finne P, Reunanen A, Stenman S. Incidence of end stage renal disease in patients with type I diabetes. JAMA 2005;294:1782-7. [PubMed]
- Deckert T, Yokoyama H, Mathiesen E, et al. Cohort study of predictive value of urinary albumin excretion for atherosclerotic vasular disease in patients with insulin dependent diabetes. BMJ 1996;312:871-4. [PubMed]
- Earle KA, Mishra M, Morocutti A, et al. Microalbuminuria asa marker of silent myocardial iscaemia in IDDM patients. Diabetologia 1996;39:854-6. [PubMed]
- Jones SL, Close CF, Mattock MB, et al. Plasma lipid and coagulation factor concentrations in insulin dependent diabetics with microalbuminuria. BMJ 1989;298:487-90. [PubMed]
- Yip J, Mattock MB, Morocutti A, et al. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet 1993;342:883-7. [PubMed]
- Berrut G, Bouhanick B, Fabbri P, et al. Microalbuminuria as a predictor of a drop in glomerular filtration rate in subjects with non-insulin-dependent diabetes mellitus and hypertension. Clin Nephrol 1997;48:92-7. [PubMed]
- Brocco E, Fioretto P, Mauer M, et al. Renal structure and function in non-insulin dependent diabetic patients with microalbuminuria. Kidney Int Suppl 1997;63:S40-4. [PubMed]
- Mattock MB, Morrish NJ, Viberti G, et al. Prospective study of microalbuminuria as predictor of mortality in NIDDM. Diabetes 1992;41:736-41. [PubMed]
- Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med 1997;157:1413-8. [PubMed]
- Yudkin JS, Forrest RD, Jackson CA. Microalbuminuria as predictor of vascular disease in non-diabetic subjects. Islington Diabetes Survey. Lancet 1988;2:530-3. [PubMed]
- Groop L, Ekstrand A, Forsblom C, et al. Insulin resistance, hypertension and microalbuminuria in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1993;36:642-7. [PubMed]
- Mykkänen L, Zaccaro DJ, Wagenknecht LE, et al. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes 1998;47:793-800. [PubMed]
- NICE guidelines CG87. Type 2 diabetes: the management of Type 2 diabetes. 2009. Available online: http://www.nice.org.uk/guidance/cg87/chapter/1-recommendations#kidney-damage
- The EUCLID Study Group. Randomised placebo controlled trial of lisinopril in normotensive patients and insulin dependent diabetes with normoalbuminuria and microalbuminuria. Lancet 1997;349:1787-92. [PubMed]
- Borch-Johnsen K, Wenzel H, Viberti GC, et al. Is screening and intervention for microalbuminuria worthwhile in patients with insulin dependent diabetes? BMJ 1993;306:1722-5. [PubMed]
- Johnston J, Paterson KR, O'Reilly DS. Estimating urinary albumin excretion rate in clinical practice. BMJ 1993;306:493-4. [PubMed]
- Gatling W, Knight C, Hill RD. Screening for early diabetic nephropathy: which sample to detect microalbuminuria? Diabet Med 1985;2:451-5. [PubMed]
- Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80:17-28. [PubMed]
- Parving HH, Andersen AR, Smidt UM, et al. Diabetic nephropathy and arterial hypertension. Diabetologia 1983;24:10-2. [PubMed]
- Hasslacher C, Stech W, Wahl P, et al. Blood pressure and metabolic control as risk factors for nephropathy in Type 1 (insulin-dependent) diabetes. Diabetologia 1985;28:6-11. [PubMed]
- Mogensen CE. Progression of nephropathy in long-term diabetics with proteinuria and effect of initial anti-hypertensive treatment. Scand J Clin Lab Invest 1976;36:383-8. [PubMed]
- Parving HH, Andersen AR, Smidt UM, et al. Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabteic nephropathy. Lancet 1983;1:1175-9. [PubMed]
- Morelli E, Loon N, Meyer T, et al. Effects of converting-enzyme inhibition on barrier function in diabetic glomerulopathy. Diabetes 1990;39:76-82. [PubMed]
- McFarlane PA. Review: ACE inhibitors delay microalbuminuria in diabetes without nephropathy and reduce mortality in diabetic nephropathy. Evid Based Med 2006;11:144. [PubMed]
- ONTARGET Investigators, Yusuf S, Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547-59. [PubMed]
- Parving HH, Brenner BM, McMurray JJ. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012;367:2204-13. [PubMed]
- Imai E, Chan JC, Ito S, et al. Effect of olmesartan on renal and cardiovascular outcome in type 2 diabetes with overt nephropathy: a multicentre, placebo, randomised-controlled study. Diabetologia 2011;54:2978-86. [PubMed]
- Hommel E, Mahiesen E, Edsberg B, et al. Acute reduction of arterial blood pressure reduces urinary albumin excretion in type 1 (insulin-dependent) diabetic patients with incipient nephropathy. Diabetologia 1986;29:211-5. [PubMed]
- Agardh CD, Garcia-Puig J, Charbonnel B, et al. Greater reduction of urinary albumin excretion in hypertensive type II diabetic patients with incipient nephropathy by lisinopril than by nifedipine. J Hum Hypertens 1996;10:185-92. [PubMed]
- Gilbert R, Phillips P, Clarke C, et al. Day-night blood pressure variation in normotensive, normoalbuminuric type I diabetic subjects. Dippers and non-dippers. Diabetes Care 1994;17:824-7. [PubMed]
- Mathiesen ER, Hommel E, Giese J, et al. Efficacy of captopril in postponing nephropathy in normotensive insulin dependent diabetic patients with microalbuminuria. BMJ 1991;303:81-7. [PubMed]
- The Microalbuminuria Captopril Study Group. Captopril reduces the risk of nephropathy in IDDM patients with microalbuminuria. Diabetologia 1996;39:587-93. [PubMed]
- O'Donnell MP, Kasiske BL, Kim Y, et al. Lovastatin inhibits proliferation of rat mesangial cells. J Clin Invest 1993;91:83-7. [PubMed]
- Tonolo G, Ciccarese M, Brizzi P, et al. Reduction of albumin excretion rate in normotensive microalbuminuric type 2 diabetic patients during long-term simvastatin treatment. Diabetes Care 1997;20:1891-5. [PubMed]
- Abe M, Maruyama N, Okada K, et al. Effects of lipid-lowering therapy with rosuvastatin on kidney function and oxidative stress in patients with diabetic nephropathy. J Atheroscler Thromb 2011;18:1018-28. [PubMed]
- Smulders YM, Van Eeden AE, Stehouwer CD, et al. Can reduction in hypertriglyceridaemia slow progression of microalbuminuria in patients with non-insulin-dependent diabetes mellitus? Eur J Clin Invest 1997;27:997-1002. [PubMed]
- Forsblom C, Hiukka A, Leinonen ES, et al. Effects of long-term fenofibrate treatment on markers of renal function in type 2 diabetes: the FIELD Helsinki substudy. Diabetes Care 2010;33:215-20. [PubMed]
- Nezu U, Kamiyama H, Kondo Y, et al. Effect of low-protein diet on kidney function in diabetic nephropathy: meta-analysis of randomised controlled trials. BMJ Open 2013;3:e002934. [PubMed]
- DCCT/EDIC research group. Effect of intensive diabetes treatment on albuminuria in type 1 diabetes: long-term follow-up of the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications study. Lancet Diabetes Endocrinol 2014;2:793-800. [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53. [PubMed]
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364-79. [PubMed]
- Microalbuminuria Collaborative Study Group, United Kingdom. Intensive therapy and progression to clinical albuminuria in patients with insulin dependent diabetes mellitus and microalbuminuria. BMJ 1995;311:973-7. [PubMed]
- Kim YS, Kim MS, Kim SI, et al. Post-transplantation diabetes is better controlled after conversion from prednisolone to deflazacort: a prospective trial in renal transplants. Transpl Int 1997;10:197-201. [PubMed]