
Wearing masks to reduce the spread of respiratory viruses: a systematic evidence mapping
Introduction
On December 31, 2019, a novel coronavirus was reported for the first time in Wuhan, China. The virus is now named by the World Health Organization (WHO) as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). On March 11, 2020, WHO characterized the coronavirus disease 2019 (COVID-19) outbreak as a pandemic (1). There is a lack of specific antiviral treatment or available vaccines that have proven to be effective for this new viral disease (2). The infected people primarily rely on symptomatic treatment and supportive care (3,4). Authorities of most countries have recommended measures such as maintaining social distancing and washing hands, which are considered extremely important measures to reduce the risk of infection (5-7). However, given the cultural differences or absence of high-quality evidence, controversies over the effectiveness, safety, and enforceability of masks worn by the public were prominent in the early stages of this global epidemic.
Most of the available research on masks focused on healthcare workers and household contacts (individuals living in a household with patients with a respiratory virus infection) (8), and data on other populations are scarce (9,10). Furthermore, there are contradictions in the research results between different study settings (such as hospitals, community, and laboratory), which prevents the decision makers from making appropriate judgments (11). Therefore, our study focused on randomized controlled trials (RCTs) of non-laboratory research or systematic reviews (SRs) including RCTs (which met our inclusion criteria of RCTs). This is because non-laboratory studies might generalize to a wider population, and RCTs and SRs, as high-quality study designs, have the highest possible quality of evidence and are an important reference value for decision makers in general (12,13). In addition, the outcomes that we mainly focused on included influenza-like illness (ILI), laboratory-confirmed respiratory infection, and self-reported infection symptoms, which are the most common judgment indicators with regard to the spread of respiratory viruses (11). The ILI was usually defined as fever >38 °C and one or more of the following symptoms: nasal discharge/congestion, cough, conjunctivitis, respiratory distress (tachypnea, retractions), sore throat, and new seizure (8).
In addition, medical and public health professionals are concerned that the improper mask use may cause other unfavorable effects (14), policymakers also urgently need relevant high-quality evidence to support policy making. In a previous study, it was suggested that many COVID-19-related studies are poorly designed, merely adding to the COVID-19 noise (13). Therefore, it is necessary to comprehensively and systematically collect, present, and analyze current high-quality design studies. Evidence mapping (EM) is a type of comprehensive evidence-based research method that systematically and rapidly collects, evaluates, organizes, and presents existing evidence (15,16). EM presents a visual overview of existing evidence in a certain research field, and clarifies the characteristics of the studies in this field from multiple dimensions (such as the types of interventions, the research population, conclusions of the research, etc.), thereby providing systematic evidence support for decision makers (17). Furthermore, EM can also help identify evidence gaps (18). Therefore, EM can be the first step to conduct SRs or the framework to inform policy development (19). However, EM does not provide details on the generation of research results or incorporate meta-analytic techniques for pooling effect estimates, which is currently perhaps the most controversial point in EM methodology (20). Currently, no EM study, based on related RCTs and SRs, exists that presents and assesses the effectiveness and adverse effects of wearing masks to control the spread of respiratory viruses. Thus, in this study, we aimed to identify, describe, and organize currently available high-quality design evidence for mask use during the spread of respiratory viruses through an EM approach and identify gaps in evidence.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/atm-20-6745).
Methods
Literature search
We searched four databases (Web of Science, Cochrane Library, EMBASE, and PubMed) on April 9, 2020. Major search terms and strategies (Appendix 1) were as follows: ("Mask"[Mesh] OR mask OR facemask OR masks OR respirator OR N95 OR FFP2 OR "personal protective equipment" OR protective devices) AND ("Respiratory Tract Infections"[Mesh] OR ILI OR infect OR influenza OR MERS OR “Middle East respiratory syndrome” OR pandemic OR parainfluenza OR “respiratory disease” OR “respiratory illness” OR “respiratory infection” OR “respiratory hygiene” OR “respiratory virus” OR SARS OR SARS-CoV-2 OR COVID-19 OR “severe acute respiratory syndrome” OR virus) AND (“random*” OR “blind*” OR “singleblind*” OR “doubleblind*” OR “trebleblind*” OR “tripleblind*”). Moreover, the WHO International Clinical Trials Registry Platform (ICTRP) Search Portal, clinical trial registry, reference lists of articles, and gray literature were searched on April 27, 2020.
Inclusion and exclusion criteria
RCTs and SRs including RCTs that evaluated the mask use as an intervention against the spread of respiratory viruses were included in the study. The inclusion criteria were as follows: (I) no restriction for participants; (II) inclusion of mask intervention the treatment or intervention group (e.g., face mask, N95 respirator, and/or medical/surgical masks); and (III) inclusion of usual practice (e.g., education without the face mask use) or medical/surgical masks in the control groups. Furthermore, when several SRs published by the same team were identified, the most recent publication was considered. The following studies were excluded: (I) duplicate reports of a study; (II) studies with insufficient data (e.g., conference abstracts); (III) non-human studies; and (IV) laboratory studies.
Study selection and data extraction
Literature screening and data extraction were performed by two independent reviewers. Different views between the two reviewers were discussed and resolved by a third independent reviewer. EndNote X9 software was used to remove duplicates. Subsequently, the title and abstract of preliminary included studies were screened by two independent reviewers. For studies that according by both reviewers should be excluded, further screening was not conducted. For studies that according to at least one reviewer should be included or if a definitive decision could not be made, the full text was further screened and the suitability for final inclusion was determined. A predesigned table was designed to conduct data extraction, and general information was extracted about the study, including publication year, the first author, and country. We also included details concerning the type of intervention, population, result, conclusion, study design, and sample size.
Quality assessment
The tool recommended by the Cochrane Handbook Version 5.1.0 (21) was used to analyze the risk of bias of the included trials based on the following factors: random sequence generation, incomplete outcome data, allocation concealment, blinding of outcome assessment, selective reporting, blinding of participants and personnel, and other bias. Each item was classified as “Yes” (“low risk of bias”), “No” (“high risk of bias”), or “Unclear” (“moderate risk of bias”). When the risk of bias for all seven factors was assessed as “low risk of bias,” the trial was assessed to have an overall “low risk of bias.” Accordingly, when one or more of the seven bias factors were assessed as high risk, the trial was assessed to have a “high risk of bias.” For other cases, the trial was assessed to have an “unclear risk.” Differences in bias assessment were resolved through discussion by two independent reviewers. Furthermore, in some cases, a third reviewer participated in the resolution of differences.
The Assessment of Multiple Systematic Reviews (AMSTAR-2) tool (22) was used to assess the methodological quality of all SRs. AMSTAR 2 consists of 16 items and each item was evaluated using “Yes”, “Partial Yes”, or “No”. The assessment process was conducted online (https://amstar.ca/Amstar_Checklist.php), the overall quality assessment results (“Critically low quality,” “Low quality,” “Moderate quality”, or “High quality”) was automatically generated. Two independent reviewers evaluated these items, and differences were resolved by discussion with a third reviewer.
Data synthesis and analysis
Currently, there is a lack of reporting guidelines or methodological guidance with regard to EM. We are, therefore, based our study on the methodology of Global Evidence Mapping (23), Campbell evidence and gap maps (24), and our previous findings (17) concerning EM and evidence and gap map methodology, and made necessary expansion on this basis (25,26). All authors have fully discussed the extension of each methodology and the construction of the framework of this article. A bubble plot was designed to display information in four dimensions as follows (27,28): (I) each bubble represents one RCT/SR and different colors represent various research populations; (II) the bubble size represents the sample size/number of RCTs included in this mapping; (III) the rating of authors’ conclusions are represented on the X-axis as “beneficial,” “probably beneficial,” “harmful,” “no effect,” and “inconclusive”; and (IV) quality assessment is represented on the Y-axis. We observed that some studies (15,27) have made meaningful explorations especially in terms of rating of the authors’ conclusions. Based on these studies, we conducted in-depth discussions and divided the conclusions into five categories considering the descriptions of both the results and conclusions of the included study, in which “beneficial” indicated that the conclusions and results reported a clear beneficial effect without major concerns regarding supporting evidence, “probably beneficial” suggested that the conclusions did not claim firm benefits despite the reported positive treatment effect or the conclusions reported a potential benefits despite the result showing no significant difference, “harmful” suggested that the conclusions and results were reported to be clearly indicative of a harmful effect, “no effect” suggested that the conclusions and results provided evidence of no differences between intervention and comparator, and “inconclusive” suggested that the results of the study were insufficient for the authors to conclude whether the intervention has a definitive or potential effect. Moreover, the judgment indicators mainly were ILI, laboratory-confirmed respiratory infection, or self-reported infection symptoms. In addition, narrative synthesis was conducted for expanding upon mapping to provide more details about the included studies. These included descriptions of the evidence gaps and adverse events.
Results
Study selection
As shown in Figure 1, a total of 7,006 studies were initially included; however, of these, 1,512 duplicates were excluded. The titles and abstracts of the remaining 5,494 studies were screened, following which 5,430 studies were deemed unsuitable for inclusion. The full texts of the remaining 64 studies were screened and another 34 articles were excluded (Table S1). Finally, 21 RCTs and nine SRs were included and analyzed.
Study characteristics
The essential information of the included studies has been shown in Table 1 (Table S2 for a more detailed summary by PICO). In total, 21 trials evaluating 18,709 individuals were included in our study. Of the selected studies, the highest proportion were conducted in China (>30%, 7/21), followed by USA (23.81%, 5/21), Canada (9.52%, 2/21), Australia (4.76%, 1/21), France (4.76%, 1/22), Germany (4.76%, 1/21), Japan (4.76%, 1/21), Saudi Arabia (4.76%, 1/21), Thailand (4.76%, 1/21), and Vietnam (4.76%, 1/21). The populations in eight trials included healthcare workers (38.10%, 8/21); seven trials, household contacts (33.33%, 7/21); two trials, students (9.52%, 2/21); two trials, exposed participants (9.52%, 2/21); one trial, crewmembers (4.76%, 1/21); and one trial, Australian pilgrims (4.76%, 1/21). Furthermore, nine SRs were included in our study; of these, three were conducted in China (33.33%) and Canada (33.33%), one in Sweden (11.11%), the UK (11.11%), Australia (11.11%), and Singapore (11.11%). The populations in three of these studies included healthcare workers (33.33%); one, household contacts (11.11%), and five, mix populations (55.56%).
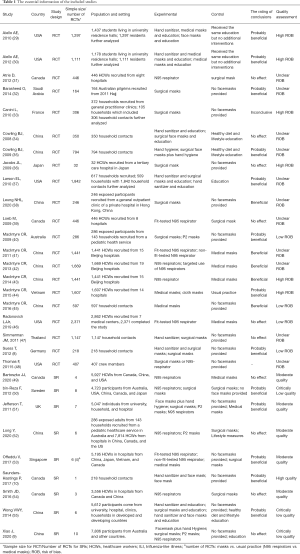
Full table
Quality assessment
A summary of the risk of bias for each included trial is shown in Figure 2. In the random-sequence generation analysis, over 80% (17/21) of trials described an adequate random-sequence generation process. Over 50% (12/21) trials described the use of sealed, opaque envelopes for allocation concealment. No one trial was selective in their data reporting. Eight trials had a “low risk of bias” regarding the blinding of outcome assessment and three trials had a “high risk of bias” in terms of blinding of participants. In addition, over 70% (15/21) of trials were found to have a “low risk of bias” in terms of incomplete outcome data. Other bias was detected in one trial.
As shown in Figure 3, according to the evaluation criteria of the latest version of AMSTAR-2, all SRs reported the components of PICO, duplicated coding for study selection and data extraction, and on comprehensive literature search, eight of the remaining items (items 2, 3, 8, 9, 11, 12, 13, and 16) were reported by over 50% SRs. Only less than 40% SRs reported Items 7, 10, 14, and 15. In particular, item 7 (provide a list of excluded studies and justify the exclusions) was reported in only one SR. In addition, only one SR (1/9) was assessed to be of “High quality”, five SRs (5/9) were assessed to be of “Moderate quality”, and three (3/9) SRs were assessed to be of “Critically Low” (Table S3).
Mapping
As shown in Figure 4, a bubble plot was designed for mapping, and four dimensions were used to visualize the RCTs and SRs (research populations, sample size/number of RCTs, the rating of conclusions, and quality assessment).
Masks vs. usual practice
As shown in Figure 4A, six SRs (9,10,50,51,53,55) evaluated the effects of wearing masks on the interruption or reduction in the spread of respiratory viruses compared with control groups (i.e., with only education and no face masks). Among them, five SRs (10,50,51,53,55) were selected as “probably beneficial” on the map suggestive of the probable effectiveness of regular masks in limiting transmission during pandemics; the effectiveness of masks and respirators in these studies was likely linked to early, consistent, and correct usage. The remaining study (9) showed “no effect” indicative of limited evidence to support the effectiveness of masks. Moreover, three SRs (9,50,55) were classified to be of “critically low quality”, two (51,53) of “moderate quality”, and one (10) of “high quality”. Overall, 83.33% SRs (5/6, involving 28 RCTs) were included in “beneficial” or “probably beneficial” categories.
As shown in Figure 4B, 14 RCTs (8,29,30,32-38,40,44,45,47) including 9,997 participants researched the effects of wearing masks on the interruption or reduction in the spread of respiratory viruses when compared to the control groups. Among these, two RCTs (38,45) with 843 participants were categorized as “beneficial” indicating that masks were effective in interrupting or reducing the spread of respiratory viruses. Eight RCTs (8,29,30,32,35,37,40,44), including 7,319 participants were categorized as “probably beneficial”, thereby indicating that masks may be helpful, and recommended wearing masks to interrupt the spread of respiratory viruses. Furthermore, three RCTs (34,36,37), including 1,529 participants were categorized as “no effect”. The remaining RCT (33) including 306 participants was found to be “inconclusive”, indicating that there was no sufficient evidence to draw a conclusion based on the research. Moreover, according to the risk of bias tool, three (8,40,45), five (29,30,33,36,44), and six (32,34,35,37,38,47) RCTs were assessed as “low risk of bias,” “high risk of bias,” and “unclear risk of bias,” respectively. In all, 71.43% RCTs (10/14, including 8162 participants) were classified into “beneficial” or “probably beneficial” categories.
N95 respirators vs. medical masks
As shown in Figure 4A, four SRs (49,52-54) evaluated the effect of N95 respirators on the interruption or reduction of the spread of respiratory viruses compared that with the effect of medical masks. Three SRs were categorized as “no effect,” thereby indicating that N95 respirators did not have a better effect compared with medical masks. Furthermore, all four SRs (49,52-54) were assessed as “moderate quality”. In all, only 25% (1/4, involving 4 RCTs) SRs was categorized under “beneficial” or “probably beneficial” categories.
As shown in Figure 4B, six RCTs (31,39,41-43,46) including 7814 participants evaluated the effect of N95 respirator on the interruption or reduction of the spread of respiratory viruses and compared that with the effect of medical masks. Among these, three RCTs (41-43), including 4,551 participants were categorized as “beneficial”, thereby suggesting that N95 respirators may be effective for interrupting or reducing the spread of respiratory viruses compared with medical masks. Furthermore, three RCTs (31,39,46) including 3,263 participants showed “no effect,” indicating similar effects between N95 respirators and medical masks. In addition, one RCT (46) was assessed as “low risk of bias;” one (43), as “high risk of bias;” and four (31,39,41,42), as “unclear risk of bias”. Overall, 50% RCTs (3/6, including 4,551 participants) were classified into “beneficial” or “probably beneficial” categories.
Adverse effects
Six trials (8,33,36,44,46,48) partially reported possible adverse effects of wearing masks, and showed that the mask groups were more likely to experience headaches during the study period, skin irritation, worsening acne, shortness of breath, and respiratory difficulties. In addition, since masks seem to affect the precise and clear transmission and reception of some aviation terms or instructions (i.e., helipad, fuel, weather) by pilots, flight nurses, layperson, dispatcher, etc., especially when the aircraft's engine is turned on, mask use may adversely affect radio communication (48). Notably, MacIntyre et al. (44) compared the efficacy of cloth masks to that of medical masks in hospital healthcare workers, and showed that participants using cloth masks (cotton, or gauze masks) showed a significantly higher rate of ILI compared with controls and suggested caution against cloth mask use.
Discussion
Summary of findings
In this EM study, concerning mask use for the prevention of the spread of respiratory viruses, we systematically searched for relevant published RCTs and SRs before April 2020. In all, 21 RCTs and nine SRs were included in this study. Among the 21 RCTs, most studies were conducted in China and the USA, and focused on the healthcare workers and household contacts. Overall, masks versus usual practice, 10 of 14 RCTs and 5 of 6 SRs were classified as “beneficial” or “probably beneficial”. Furthermore, regarding N95 respirators versus medical masks, 3 of 6 RCTs were classified as “beneficial”; however, 75% of SRs showed that there was no significant difference between groups. In addition, six RCTs reported adverse effects of wearing masks, with one RCT implying that the cloth mask reuse may increase the risk of infection.
In terms of conclusion ratings, when comparing data between with and without masks, most included RCTs, as well as SRs, showed “beneficial” or “probably beneficial” effects of masks, with a higher number of participants wearing masks grouped in “beneficial” and “probably beneficial” categories compared to any other category (8,162 vs. 1,835), thereby suggesting that masks may have a positive effect on interrupting or reducing the spread of respiratory viruses, especially for healthcare workers, all relevant studies included show “probably beneficial” effects of masks. However, when comparing the outcomes with N95 respirators and those with medical masks, over 70% of SRs showed “no effect,” whereas 50% of RCTs showed “beneficial” effects. Therefore, we were unable to draw a definitive conclusion on whether the N95 respirator is a better or worse choice than medical masks based on the current evidence. Thus, more relevant high-quality studies are needed for making this conclusion. In addition, among the 10 studies included, the subjects of nine studies were healthcare workers. Combined, the results of these studies largely showed that there were conflicting results regarding whether healthcare workers should wear N95 respirators or medical masks. Moreover, the reasons for this inconformity may be as follows. First, we ascertained the rating of conclusions (“beneficial”, “probably beneficial”, “harmful”, “no effect” and “inconclusive”) based on the descriptions of both the results and conclusions of the study; the conclusions of most RCTs considered the study design, intervention compliance, and sample size. Thus, the conclusions may be inconsistent with the statistical results. However, the conclusions of SRs depended more on the statistical effect (56-60). Second, the sample sizes of RCTs categorized into “beneficial” or “probably beneficial” categories and those of RCTs categorized into the “no effect” category were similar (4,551 vs. 3,263) (60).
Regarding the adverse effects of wearing masks, many experts and studies have indicated that given that complete elimination of COVID-19 does not seem likely in the near future, protective measures, such as maintaining social distancing and wearing masks may be necessary for a prolonged time. Furthermore, according to searched studies, insufficient high-quality design research was available that reported on the adverse effects of prolonged mask use. Among the 21 included RCTs, six reported possible adverse effects of prolonged mask use, such as headaches, skin irritation, and respiratory difficulties. In particular, one RCT implied that cloth mask reuse may increase the risk of an infection (61). It is noteworthy that cloth masks are commonly used in developing countries, although many non-standard practices around cleaning and cloth mask reuse have evolved. Furthermore, given the COVID-19 situation, many developed countries are widely using cloth masks (44,62). This should draw the attention of the researchers and decision makers. Moreover, there is a lack of RCTs that systematically evaluate the adverse effects of the prolonged wear of masks. This may be because historically, the need to study this has been limited, given that very few pandemics requiring the mask use have been reported. Accordingly, there is limited literature on prolonged mask use, making it difficult to implement RCTs (63-66). A non-RCT reported that masks, especially N95 respirators, affected air intake, thereby decreasing the respiratory efficiency and increasing the respiratory burden (61), and this may affect normal life and even be life threatening for vulnerable populations, such as children, pregnant women, the elderly population, and individuals with chronic diseases or those performing high-intensity exercise. Thus, related RCTs should focus on developing a high-quality study design for evaluating this. In addition, for individuals with poor hearing or those who rely on lip reading, whether masks will significantly affect work efficiency and daily communication is worth further research.
The conclusion of this mapping study should be interpreted with caution because of the quality of the included studies. Combined, the quality of the included RCTs was relatively low as only four (4/21, 19.05%) were assessed to have a “low risk of bias”. Furthermore, allocation concealment and outcome assessment blinding were weak links in the design and reporting of the included RCTs, which may affect the authenticity of the reported observations. Moreover, three SRs (3/9, 33.33%) assessed were of “critically low quality”. Particularly, only one SR included “list of excluded studies and justification for exclusion”, which needs the attention of researchers in the future.
Evidence gaps and future directions
Current evidence of high-quality design research concerning the mask use may be insufficient to deal with a second impact of such a pandemic in the future. First, in our study, EM showed that most studies focused on the effectiveness of masks compared with usual practice than that of N95 respirators compared with medical masks. Accordingly, further research is required for differential ratings of conclusions between SRs and RCTs in terms of effectiveness of N95 respirators compared with medical masks, especially for healthcare workers. Second, over 70% of RCTs focused on healthcare workers and household contacts, and the study of populations in places of gathering, such as students and company staff, was limited. Third, high-quality studies evaluating the adverse events of the prolonged wear of masks are of utmost importance, especially in special populations (such as children, pregnant women, the elderly population, and individuals with chronic diseases, poor hearing, patients who rely on lip reading, or those performing high-intensity exercise), and cases of special reactions (such as the obstruction of vision, skin allergy and sudden death). Fourth, given difficulty in accessing medical masks for many individuals during the pandemic, cloth masks were used as a substitute. However, there is currently only one RCT evaluating the effects of using a cloth mask, which reported that the cloth mask reuse showed a “harmful effect” and may increase the risk of an infection. Accordingly, additional high-quality studies are needed in the future. Fifth, optimal settings and exposure circumstances for populations to use masks should be investigated. For example, high-quality research is needed to explore the effects of wearing masks outdoors as well as indoors.
Strengths and limitations
Compared with other studies (9,11), our research systematically searched and included relevant high-quality study designs (RCTs and SRs), and used bubble charts to visually present the existing research from multiple important dimensions. Moreover, we ascertained the rating of conclusions based on the descriptions of both the results and conclusions of the studies, which may avoid the uncertainty caused by policy recommendations determined based on only the result or conclusion of studies in a sense (56,58,63). In addition, we found evidence gaps, which not only are instructive for future research and for avoiding the wastage of academic resources but are also of great significance to policy makers. Some limitations of this study should be mentioned. First, we did not include other study designs (such as cohort studies, and case analysis); however, RCTs and SRs usually provide the highest quality evidence for decision-making. Second, our findings are only based on publications before the search date (April 9, 2020). With the emergence of newly related studies, regular updates of the existing results will be done in two years. Third, we did not perform sensitivity analysis, heterogeneity analysis, etc., because unlike SRs, these are not performed in EMs.
Conclusions
The current evidence of high-quality design research concerning mask use may be insufficient to deal with a second impact of such a pandemic in the future. Overall, masks may be effective in interrupting or reducing the spread of respiratory viruses. However, the study conclusions on the effectiveness of N95 respirators over medical masks are contradictory, especially for healthcare workers, and high-quality design evidence for mask use by a special population (such as students and company employees) is rare, and this requires further research. In addition, it is noteworthy that a few adverse effects of wearing masks have been systematically reported in existing high-quality design evidence. Accordingly, many high-quality studies are of utmost importance to assess the impact of the prolonged wear of masks on vulnerable populations and to assess the possible adverse events. Finally, in view of the current research, cloth mask reuse may aggravate the spread of respiratory infection, which needs to be further evaluated.
Acknowledgments
Funding: This study was supported by the Major Project of the National Social Science Fund of China: “Research on the Theoretical System, International Experience and Chinese Path of Evidence-based Social Science” (Project No. 19ZDA142).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-6745
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-6745
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6745). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
Organization WH 2020 .- Zhang X, Tan R, Lam WC, et al. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Extension for Chinese Herbal Medicines 2020 (PRISMA-CHM 2020). Am J Chin Med 2020;48:1279-313. [Crossref] [PubMed]
- Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty 2020;9:29. [Crossref] [PubMed]
- Cascella M, Rajnik M, Cuomo A, et al. Features, evaluation and treatment coronavirus (COVID-19). Statpearls [internet]. StatPearls Publishing; 2020.
- Organization WH. Considerations for quarantine of individuals in the context of containment for coronavirus disease (COVID-19): interim guidance, 29 February 2020: World Health Organization 2020.
- Organization WH. Management of ill travellers at points of entry–international airports, seaports and ground crossings–in the context of COVID-19 outbreak: interim guidance, 16 February 2020: World Health Organization 2020.
- Organization WH. Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19): interim guidance, 19 March 2020: World Health Organization 2020.
- Suess T, Remschmidt C, Schink SB, et al. The role of facemasks and hand hygiene in the prevention of influenza transmission in households: results from a cluster randomised trial; Berlin, Germany, 2009-2011. BMC Infect Dis 2012;12:26. [Crossref] [PubMed]
- Xiao J, Shiu EYC, Gao H, et al. Nonpharmaceutical Measures for Pandemic Influenza in Nonhealthcare Settings-Personal Protective and Environmental Measures. Emerg Infect Dis 2020;26:967. [Crossref] [PubMed]
- Saunders-Hastings P, Crispo JAG, Sikora L, et al. Effectiveness of personal protective measures in reducing pandemic influenza transmission: A systematic review and meta-analysis. Epidemics 2017;20:1-20. [Crossref] [PubMed]
- MacIntyre CR, Chughtai AA. Facemasks for the prevention of infection in healthcare and community settings. BMJ 2015;350:h694. [Crossref] [PubMed]
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003;290:1624-32. [Crossref] [PubMed]
- Glasziou PP, Sanders S, Hoffmann T. Waste in covid-19 research. BMJ 2020;369:m1847. [PubMed]
- Feng S, Shen C, Xia N, et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med 2020;8:434-6. [Crossref] [PubMed]
- Anaya MM, Franco JVA, Ballesteros M, et al. Evidence mapping and quality assessment of systematic reviews on therapeutic interventions for oral cancer. Cancer Manag Res 2018;11:117-30. [Crossref] [PubMed]
- Hetrick SE, Parker AG, Callahan P, et al. Evidence mapping: illustrating an emerging methodology to improve evidence-based practice in youth mental health. J Eval Clin Pract 2010;16:1025-30. [Crossref] [PubMed]
- Li Y, Li X, Li R, et al. Generation and reporting of evidence mapping. Chinese Journal of Evidence-Based Medicine 2020;20:1098-103.
- Gaarder M, Snilstveit B, Vojtkova M, et al. Evidence Gap Maps: A Tool for Promoting Evidence-Informed Policy and Prioritizing Future Research. 2013.
- Sawicki CM, Livingston KA, Ross AB, et al. Evaluating Whole Grain Intervention Study Designs and Reporting Practices Using Evidence Mapping Methodology. Nutrients 2018;10:1052. [Crossref] [PubMed]
- Singh K, Ansari M, Galipeau J, et al. An Evidence Map of Systematic Reviews to Inform Interventions in Prediabetes. Canadian Journal of Diabetes 2012;36:281-91. [Crossref]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 2011;343:d5928. [Crossref] [PubMed]
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [Crossref] [PubMed]
- Bragge P, Clavisi O, Turner T, et al. The global evidence mapping initiative: scoping research in broad topic areas. BMC Med Res Methodol 2011;11:92. [Crossref] [PubMed]
- Welch V, Howe T, Marcus S, et al. PROTOCOL: Health, social care and technological interventions to improve functional ability of older adults: Evidence and gap map. Campbell Systematic Reviews 2019;15. [Crossref]
- Yang K. Evidence-based social science: the origin, development and prospects. Library and Information 2018:1-10.
- Yang K, Li X, Bai Z. Research methods of Evidence-based social science: Systematic review and meta-analysis. Lanzhou: Lanzhou University Press; 2018.
- Ballesteros M, Montero N, Lopez-Pousa A, et al. Evidence mapping based on systematic reviews of therapeutic interventions for gastrointestinal stromal tumors (GIST). BMC Med Res Methodol 2017;17:135. [Crossref] [PubMed]
- Miake-Lye IM, Mak S, Lee J, et al. Massage for Pain: An Evidence Map. J Altern Complement Med 2019;25:475-502. [Crossref] [PubMed]
- Aiello AE, Murray GF, Perez V, et al. Mask use, hand hygiene, and seasonal influenza-like illness among young adults: a randomized intervention trial. J Infect Dis 2010;201:491-8. [Crossref] [PubMed]
- Aiello AE, Perez V, Coulborn RM, et al. Facemasks, hand hygiene, and influenza among young adults: a randomized intervention trial. PLoS One 2012;7:e29744 [Crossref] [PubMed]
- Atrie D, Worster A. Surgical mask versus N95 respirator for preventing influenza among health care workers: a randomized trial. CJEM 2012;14:50-2. [Crossref] [PubMed]
- Barasheed O, Almasri N, Badahdah AM, et al. Pilot Randomised Controlled Trial to Test Effectiveness of Facemasks in Preventing Influenza-like Illness Transmission among Australian Hajj Pilgrims in 2011. Infect Disord Drug Targets 2014;14:110-6. [Crossref] [PubMed]
- Canini L, Andreoletti L, Ferrari P, et al. Surgical mask to prevent influenza transmission in households: a cluster randomized trial. PLoS One 2010;5:e13998 [Crossref] [PubMed]
- Cowling BJ, Fung RO, Cheng CK, et al. Preliminary findings of a randomized trial of non-pharmaceutical interventions to prevent influenza transmission in households. PLoS One 2008;3:e2101 [Crossref] [PubMed]
- Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: A cluster randomized trial. Ann Intern Med 2009;151:437-46. [Crossref] [PubMed]
- Jacobs JL, Ohde S, Takahashi O, et al. Use of surgical face masks to reduce the incidence of the common cold among health care workers in Japan: a randomized controlled trial. Am J Infect Control 2009;37:417-9. [Crossref] [PubMed]
- Larson EL, Ferng YH, Wong-McLoughlin J, et al. Impact of non-pharmaceutical interventions on URIs and influenza in crowded, urban households. Public Health Rep 2010;125:178-91. [Crossref] [PubMed]
- Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 2020;26:676-80. [Crossref] [PubMed]
- Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA 2009;302:1865-71. [Crossref] [PubMed]
- MacIntyre CR, Cauchemez S, Dwyer DE, et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis 2009;15:233-41. [Crossref] [PubMed]
- MacIntyre CR, Wang Q, Cauchemez S, et al. A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respir Viruses 2011;5:170-9. [Crossref] [PubMed]
- MacIntyre CR, Wang Q, Seale H, et al. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med 2013;187:960-6. [Crossref] [PubMed]
- MacIntyre CR, Wang Q, Rahman B, et al. Efficacy of face masks and respirators in preventing upper respiratory tract bacterial colonization and co-infection in hospital healthcare workers. Prev Med 2014;62:1-7. [Crossref] [PubMed]
- MacIntyre CR, Seale H, Dung TC, et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open 2015;5:e006577 [Crossref] [PubMed]
- MacIntyre CR, Zhang Y, Chughtai AA, et al. Cluster randomised controlled trial to examine medical mask use as source control for people with respiratory illness. BMJ Open 2016;6:e012330 [Crossref] [PubMed]
- Radonovich LJ Jr, Simberkoff MS, Bessesen MT, et al. N95 Respirators vs Medical Masks for Preventing Influenza Among Health Care Personnel: A Randomized Clinical Trial. JAMA 2019;322:824-33. [Crossref] [PubMed]
- Simmerman JM, Suntarattiwong P, Levy J, et al. Findings from a household randomized controlled trial of hand washing and face masks to reduce influenza transmission in Bangkok, Thailand. Influenza Other Respir Viruses 2011;5:256-67. [Crossref] [PubMed]
- Thomas F, Allen C, Butts W, et al. Does wearing a surgical facemask or N95-respirator impair radio communication? Air Med J 2011;30:97-102. [Crossref] [PubMed]
- Bartoszko JJ, Farooqi MAM, Alhazzani W, et al. Medical Masks vs N95 Respirators for Preventing COVID-19 in Health Care Workers A Systematic Review and Meta-Analysis of Randomized Trials. Influenza Other Respir Viruses 2020;14:365-73. [Crossref] [PubMed]
- Bin-Reza F, Lopez Chavarrias V, Nicoll A, et al. The use of masks and respirators to prevent transmission of influenza: A systematic review of the scientific evidence. Influenza Other Respir Viruses 2012;6:257-67. [Crossref] [PubMed]
- Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev 2011;2011:CD006207 [Crossref] [PubMed]
- Long Y, Hu T, Liu L, et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med 2020;13:93-101. [Crossref] [PubMed]
- Offeddu V, Yung CF, Low MSF, et al. Effectiveness of Masks and Respirators Against Respiratory Infections in Healthcare Workers: A Systematic Review and Meta-Analysis. Clin Infect Dis 2017;65:1934-42. [Crossref] [PubMed]
- Smith JD, MacDougall CC, Johnstone J, et al. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: A systematic review and meta-analysis. CMAJ 2016;188:567-74. [Crossref] [PubMed]
- Wong VWY, Cowling BJ, Aiello AE. Hand hygiene and risk of influenza virus infections in the community: A systematic review and meta-analysis. Epidemiol Infect 2014;142:922-32. [Crossref] [PubMed]
- Ge L, Tian J-h, Li Y-n, et al. Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta-epidemiological study. J Clin Epidemiol 2018;93:45-55. [Crossref] [PubMed]
- Tian J, Zhang J, Ge L, et al. The methodological and reporting quality of systematic reviews from China and the USA are similar. J Clin Epidemiol 2017;85:50-8. [Crossref] [PubMed]
- Xiu-xia L, Ya Z, Yao-long C, et al. The reporting characteristics and methodological quality of Cochrane reviews about health policy research. Health Policy 2015;119:503-10. [Crossref] [PubMed]
- Yao L, Sun R, Chen Y-L, et al. The quality of evidence in Chinese meta-analyses needs to be improved. J Clin Epidemiol 2016;74:73-9. [Crossref] [PubMed]
- Delgado-Rodríguez M, Sillero-Arenas M. Systematic review and meta-analysis. Med Intensiva 2018;42:444-53. [Crossref] [PubMed]
- Tong PSY, Kale AS, Ng K, et al. Respiratory consequences of N95-type Mask usage in pregnant healthcare workers-a controlled clinical study. Antimicrobial Resistance and Infection Control 2015;4:48. [Crossref] [PubMed]
- CDC T. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/diy-cloth-face-coverings.html. 2020.
- Bhargava A. Randomized controlled experiments in health and social sciences: some conceptual issues. Econ Hum Biol 2008;6:293-8. [Crossref] [PubMed]
- Lina B. History of Influenza Pandemics. 2008. p. 199-211.
- Mossad SB. Influenza update 2018–2019: 100 years after the great pandemic. Cleveland Clinic Journal of Medicine 2018;85:861-9. [Crossref] [PubMed]
- Qin Y, Zhao M, Tan Y, et al. History of influenza pandemics in China during the past century. Zhonghua liu xing bing xue za zhi 2018;39:1028-31. [PubMed]






