The promise of lung master protocol for squamous cell carcinoma: one trial to rule them all, one trial to find them…?
For almost a decade, the euphoria associated with the finding of genomic targets in lung adenocarcinoma overshadowed the fact that no advance had been made in the other common subtypes of non-small cell lung cancer (NSCLC). One of those—squamous cell carcinoma (SCC) of the lung—is still a very common disease in communities that have high levels of cigarette smoking. Even in communities with smoking rates less than 20%, SCC still continues to contribute from one quarter to one third of cases. Moreover due to increased application of lineage-specific immunohistochemical markers to poorly differentiated NSCLC, many cases of previously diagnosed “large cell carcinoma” or “NSCLC, not otherwise specified” found to positive with either p40 or p63, are now diagnosed as SCC, according to the recently published 2015 World Health Organisation of Lung Tumours, which has expanded the definition of SCC to include both tumours with either morphologic or immunohistochemical evidence of squamous differentiation. In some series this may add up to another 10% to the SCC prevalence rates.
The years of nihilism appeared to end when Weiss et al. (1) identified frequent occurrences of FGFR1 amplification in 10-22% of cases in a very large collaboration between Europe countries, Australia and several cities in the US. In animal models and cell lines, FGFR1-amplified SCC was shown to regress in response to an investigational inhibitor. To add to the optimism, several agents with known FGFR1 inhibitory activity already existed, albeit as just one of several targets including other FGFR family members, VEGF receptors and PDGFR. This perhaps became a handicap, as variable potencies against FGFR1 and difficulties attracting Pharma support frustrated lung cancer centres of excellence in their quest to conduct clinical trials.
Other targets followed, including DDR2 mutation (a target for dasatinib), reported by Hammerman et al. (2,3) in about 2% of SCC. Further targets such as PIK3CA mutation, CDK4 amplification and mutations of CCND1-3 (4) now have potential active targeted drug therapies.
Collectively, the existing potential targets cover an estimated 35-45% of SCC cases, but the problem with all of these targets is that they correspond to such a small subgroup of NSCLC that trial screening costs are prohibitive and potential recruits are rare. This is the specific dilemma that the Lung Master Protocol (Lung-MAP) was designed to solve (5). Patients with advanced SCC are screened by next generation sequencing (NGS) techniques and immunohistochemistry (IHC) for known biomarkers. If a biomarker is discovered that matches one of the targeted therapies, they are assigned to that sub-study and randomized to receive standard chemotherapy or the new targeted agent. Patients are allowed to have received one line of standard chemotherapy before enrolment. If they don’t match any target, they are randomized to immunotherapy or chemotherapy.
Perhaps the most breathtaking advance of Lung-MAP is the fact that it managed to bring together the competing pharmaceutical companies, government and philanthropic funding and NGS platforms that would have been unthinkable even a decade ago. The leaders from the southwest oncology group (SWOG) and the other participating institutions should be congratulated for this achievement alone.
In essence, Lung-MAP is an umbrella trial where every recruit has comprehensive genomic analysis so that they can be placed into a specific molecular target group, or to an “unmatched’ group, for those without a known or relevant target. Recruits are then randomized to investigational targeted therapy or a defined standard therapy. Patients without a defined target are randomized to an inhibitor of programmed death ligand 1 (PD-L1) or chemotherapy. The basic schema is shown in Figure 1.
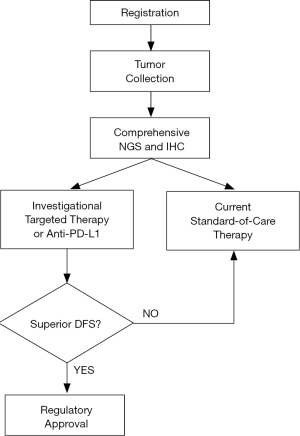
Most importantly, Lung-MAP provides a rapid pathway for FDA approval of a drug for its appropriate indication as the phase II study continues as a phase III study. Once a drug is proved safe, if a sub-trial is positive for improved disease-free survival, the drug is moved through the regulatory hurdles. A new drug and target combination can then be rolled into Lung-MAP and the trial continues. This leads to the minimum time lag to translate evidence into practice.
The advantages of Lung-MAP are manifold, including:
As of the beginning of this year, 400 sites had been activated across most states of the US. One of the sub-studies had to be closed early due to the withdrawal of rilotumumab by Amgen. This did not affect the Lung-MAP study overall due to its modular nature and the other four sub-studies have continued as planned. Another relevant development was the recent FDA approval of the PD-1 inhibitor nivolumab for SCC of the lung. Given that Lung-MAP patients that do not match to a specific biomarker are treated with the PD-L1 inhibitor MEDI4736, this may have the potential to cannibalize recruits to the newly approved therapy. Of course, the sub-study could be altered to randomize between the MEDI4736 and nivolumab, but that may prove too difficult for company boards.
The real challenge for Lung-MAP may actually come out of its own success. As new drugs are found effective, they will increasingly need to be tested as monotherapy against combined therapy to prevent resistance or to increase response rates. The introduction of nivolumab as a standard therapy may require more complex standard-of-care arms. For example, chemo-naïve patients may be given first-line chemotherapy, but those who have already received first-line may have to be placed on nivolumab (or in future, MEDI4736) as a control arm.
We look forward with renewed optimism that the success of targeted therapy in lung adenocarcinoma will be repeated in SCC. We also look forward to this clinical trial model being rolled out to other countries and to other tumour subtypes. Further details can be found on the Lung-MAP website (http://www.lung-map.org).
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by the Section Editor Zheng Yan, MD & PhD (Department of Thoracic Surgery, Henan Cancer Hospital/The Affiliated Cancer Hospital of Zhengzhou University, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Weiss J, Sos ML, Seidel D, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med 2010;2:62ra93. [PubMed]
- Hammerman PS, Lawrence MS, Voet D, et al. Comprehensive genomic characterization of squamous cell lung cancers. Cancer Genome Atlas Research Network. Nature 2012;489:519-25. [PubMed]
- Hammerman PS, Sos ML, Ramos AH, et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov 2011;1:78-89. [PubMed]
- Musgrove EA, Caldon CE, Barraclough J, et al. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 2011;11:558-72. [PubMed]
- Herbst RS, Gandara DR, Hirsch FR, et al. Lung Master Protocol (Lung-MAP)-A Biomarker-Driven Protocol for Accelerating Development of Therapies for Squamous Cell Lung Cancer: SWOG S1400. Clin Cancer Res 2015;21:1514-24. [PubMed]


