Ex situ reimplantation technique, in central lung tumors
Introduction
Non-small-cell lung cancer confined to the lung is best treated with pulmonary resection, if possible (1). Centrally placed tumors can involve structures that preclude an isolated lobectomy and require a more extended resection meaning pneumonectomy. The parenchyma-sparing resection is most often performed in patients with impaired preoperative lung or cardiovascular function who would not be able to tolerate a pneumonectomy. Because it is considered a more complex procedure and a relevant incidence of bronchial complications has been reported (2) it has not been applied widely.
In the present study, we retrospectively analyzed our experiences of the reimplantation technique in the treatment of central lung tumours of the upper lobe (stage III). We described the procedure of ex situ reimplantation technique without adding selection bias. The objective of the present retrospective analysis was to characterize the indications, patient demographics, morbidity, mortality, and late outcomes over time in patients who underwent this kind of reimplantation technique.
Material and methods of ex-situ technique of lung reimplantation
We present 9 patients mean age 62.6+16.2 years (7 males/2 females) underwent ex situ reimplantation due to extensive lung tumor of upper lobes during a period of 20 years [1993-2014].
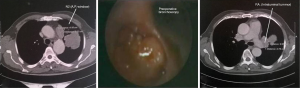
In these patients due to extensive infiltration (patient 1) or due to tumor size (patients 2,3,4) or due to the extensive involvement of the lymphatics of the upper bronchi (patients 6,8), or of the vessels of the upper lobe (patients 5,7,9) any kind of lobectomy or in vivo lobe surgery showed unfeasible (Figure 1). In such cases, the decision by the surgeon was the ex situ reimplantation technique (Table 1).
Full table.
After heparinization, a radical pneumonectomy was first performed. The pulmonary artery and the lower pulmonary veins were severed and legated, provisioning for a long enough stump of the pulmonary veins attached to the lung. Subsequently, the entire lung was immersed in Ringer’s solution (temperature 4 degrees centigrade) and bench surgery was performed. The involved upper (or upper-middle) lobes with involved lymph nodes were resected, thus leaving the healthy lower lobe of the lung. The origins of the pulmonary vessels and the bronchus were prepared.
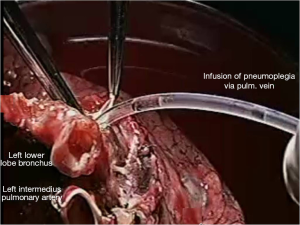
Pneumoplegia solution, named “Papworth pneumoplegia”, was administered (1,473 mL) through catheterization of the pulmonary artery and vein stumps (ante grade and retrograde) along with 250 mL of prostaglandin E1. Preparation of prostaglandin was made by adding of 0.5 mg of PGE1 in 10 mL of normal saline (NS) solution (3). After shaking of this solution 40 mL more of NS were added. Finally, a 200 mL solution in NS was prepared in order to achieve a concentration of 0.2 mg% of PGE1 (Figures 2,3).
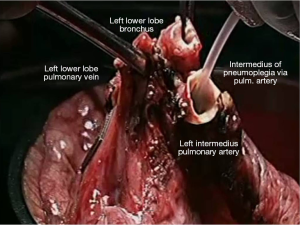
Reimplantation of the lower lobe was performed as described below.
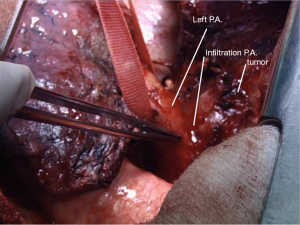
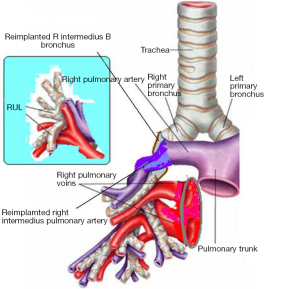
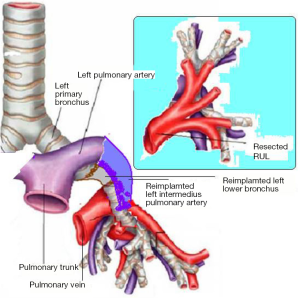
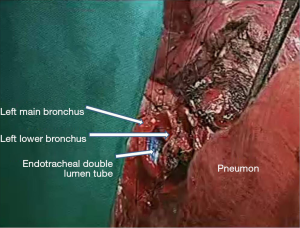
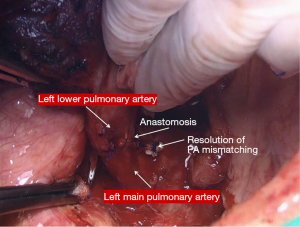
In the right side (Figure 4), implantation involved the anastomosis of lower pulmonary vein in the site of the cuff of left atrium using prolene 4-0, followed by suturing the stump of the intermedius pulmonary artery to the right main pulmonary artery, using prolene 5-0. The bronchial stumps—intermedius bronchus to the right main bronchus—were stitched together with prolene 4-0m, at the end of the procedure. On the left side (Figure 5), the sequence differed; the pulmonary vein was anastomosed first, followed by the bronchial stumps (Figure 6) and finally by the pulmonary artery (Figure 7). This is due to the different anatomical relation of the artery to the bronchus left and right (Figures 8,9).
Results
In all cases of ex-situ reimplantation, extracorporeal circulation hardware was readily available; in fact, in 3 cases (patients No.1, 3, 7) it was employed due to hemodynamic instability. In two of those cases a femoral-femoral partial bypassing performed. In the other one patient we used extracorporeal membrane oxygenator (ECMO) with partial bypass; a special venous catheter inserted along to the length of the lumen of inferior vena cava.
The graft ischemia time was 70.2+8.4 hours ranged between 55 and 80 minutes (Table 1).
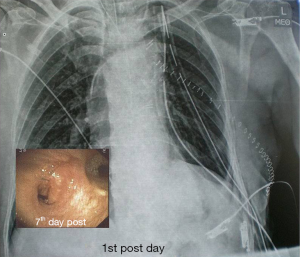
There were no significant postoperative problems during X-ray examination at 1st day and bronchoscopy reevaluation at the 7th day. Long term follow up ranged from 6 to 60 months (Table 1).
Discussion
In some patients who underwent reimplantation surgery, the upper lobectomy or the upper-middle bilobectomy may have more or specific anatomic difficulties increasing the risk of complications. The resection of this kind of tumors can be considered at bench surgery, followed by reimplantation of the remaining healthy lower lobe with bronchial, arterial or venous reconstruction. This ex situ procedure runs with or without circulatory blood pump (CBP) or with ECMO depending on the heamodynamic stability of the patient after the pneumonectomy.
Appropriate lung preservation may provide less ischemic damage and sufficient time to perform the pathological examination of the bronchial and arterial surgical margins. In addition, in agreement with others (4) we believe that the ex situ segmental graft resection may be safer and easier than the similar technique of in situ resection. It can be performed with a favorable visual field without excessive bleeding and tumor manipulation, as the cancerous lung is completely removed from the patient’s body and can be easier inspected and treated.
The major factors that play a role for the survival of initially resected and then re-implanted lung graft, are: (I) the ischemia time of the re-implanted lobe; (II) proper use of pneumoplegia solutions, use of prostaglandin E1, heparin; (III) the occurrence of pulmonary vein thrombosis; (IV) the bronchial anastomosis.
The ischemia time of the re-implanted lobe
We know that the prevalence of ischemia reperfusion injury to the re-implanted lobe increased geometrically after one hour of ischemia (5). In fact, the average reported ischemic time was from 153 min (6) to 120 min (7). These data are in accordance with the complications that described. In our cases the ischemic time ranges from 65 to 80 min (8). It is important that surgeons are well-trained in order to achieve the lowest time possible.
Proper use of pneumoplegia solutions, use of prostaglandin E1, heparin
All authors noted the importance of preventing ischemia–reperfusion injury with the lung preservation solution. Proper lung preservation was recommended in cases in which the warm ischemic time would exceed 1 h (9).
Some authors suggested the use of cold low-potassium dextran glucose lung preservation solution for ante grade followed by retrograde pulmonary arterial flushes. In our technique the resected lung was immersed in Ringer’s solution (temperature 4 degrees centigrade) and then infused with Papworth pneumoplegia with prostaglandin E1. This process was the same as in lung transplantation (10).
The occurrence of pulmonary vein thrombosis
Known factors predisposing to pulmonary vein thrombosis are the decreased blood outflow into the resected lobe, stenosis or angulation of the anastomosis, vascular compression, inadequate anticoagulant therapy and the lung ischemia-reperfusion syndrome.
Vascular complications associated with reconstruction, although uncommon, may further diminish if systemic and local heparinization is performed during flow interruption. Anticoagulant therapy for patients who have received pulmonary artery reconstruction remains controversial (11,12). It was suggested that 3,125 U of intraoperative heparin should be safe and it is unnecessary to use any more anticoagulation postoperatively (13). We used intravenous application of heparin (300 U/kg body weight per day) as a standard therapeutic regimen in all patients for 5 days after surgery and we observed no thrombosis.
The bronchial anastomosis
Pulmonary complications such as deficient healing of bronchus are to be expected in this type of patients (14). Higher frequency of sputum retention due to disruption of ciliary clearance secondary to bronchial circular suture also characterizes this type of major surgery, requiring intense respiratory physiotherapy.
For these reasons, postoperatively, all patients were transferred to our intensive care unit (ICU) and were treated by inhalation of racemic epinephrine to minimize mucosal edema followed by aggressive physiotherapy to achieve adequate drainage of bronchial secretions. Early mobilization of the patient was started as soon as possible.
In conclusion, auto-transplantation should be considered as a safe option for the appropriate patient with lung cancer (15). The ex situ separation of the cancerous lobes is technically feasible and allows extensive pulmonary resection while minimizing the loss of pulmonary reserve (16). The required surgical expertise, lung preservation techniques and the time shortening of the lung graft ischemia significantly decrease the morbidity and mortality rates and render this procedure preferable to pneumonectomy.
Acknowledgements
We want to dedicate this work to our teacher Professor Panagiotis Spyrou director and founder of Cardiothoracic Center and Transplant Unit of General Hospital “G. Papanikolaou” of Northern Greece. The authors would like to thank Dr. Dimitri Filippou for the figures that he provided.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pearson FG, Cooper JD, Deslauriers J, et al, editors. Thoracic surgery 2nd ed. New York: Churchill Livingstone 2002:837-47.
- Tedder M, Anstadt MP, Tedder SD, et al. Current morbidity, mortality, and survival after bronchoplastic procedures for malignancy. Ann Thorac Surg 1992;54:387-91. [PubMed]
- Venuta F, Rendina EA, Bufi M, et al. Preimplantation retrograde pneumoplegia in clinical lung transplantation. J Thorac Cardiovasc Surg 1999;118:107-14. [PubMed]
- Martin-Ucar AE, Chaudhuri N, Edwards JG, et al. Can pneumonectomy for non-small cell lung cancer be avoided? An audit of parenchymal sparing lung surgery. Eur J Cardiothorac Surg 2002;21:601-5. [PubMed]
- de Perrot M, Liu M, Waddell TK, et al. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med 2003;167:490-511. [PubMed]
- Jiang F, Yin R, Wang Z, et al. Transitory blocking of pulmonary artery and veins as a novel strategy in pulmonary surgery: an experimental study in a rabbit model. Eur Surg Res 2010;44:125-32. [PubMed]
- Oto T, Kiura K, Toyooka S, et al. Basal segmental auto-transplantation after pneumonectomy for advanced central lung cancer. Eur J Cardiothorac Surg 2012;42:579-81. [PubMed]
- Xu L, Hu ZD, Jiang F, et al. Transient blocking of pulmonary artery and veins for surgical treatment of stage III central lung cancer. Chin J Thorac Cardiovasc Surg 2007;23:189-92.
- Oto T. Lung transplantation from donation after cardiac death (non-heart-beating) donors. Gen Thorac Cardiovasc Surg 2008;56:533-8. [PubMed]
- Mitilian D, Sage E, Puyo P, et al. Techniques and results of lobar lung transplantations. Eur J Cardiothorac Surg 2014;45:365-9. [PubMed]
- Rendina EA, Venuta F, De Giacomo T, Ciccone AM, Moretti M, Ruvolo G, et al. Sleeve resection and prosthetic reconstruction of the pulmonary artery for lung cancer. Ann Thorac Surg 1999;68:995-1001; discussion 1001-2. [PubMed]
- Alifano M, Cusumano G, Strano S, et al. Lobectomy with pulmonary artery resection: morbidity, mortality, and long-term survival. J Thorac Cardiovasc Surg 2009;137:1400-5. [PubMed]
- Yin R, Xu L, Ren B, et al. Clinical experience of lobectomy with pulmonary artery reconstruction for central non-small-cell lung cancer. Clin Lung Cancer 2010;11:120-5. [PubMed]
- Morgan E, Lima O, Goldberg M, et al. Improved bronchial healing in canine left lung reimplantation using omental pedicle wrap. J Thorac Cardiovasc Surg 1983;85:134-9. [PubMed]
- Jiang F, Xu L, Yuan FL, et al. Lung autotransplantation technique in the treatment for central lung cancer of upper lobe. J Thorac Oncol 2008;3:609-11. [PubMed]
- Wallinder A, Ricksten SE, Silverborn M, et al. Early results in transplantation of initially rejected donor lungs after ex vivo lung perfusion: a case-control study. Eur J Cardiothorac Surg 2014;45:40-4; discussion 44-5. [PubMed]