Risk-reducing strategies for repair of complex ascending aortic false aneurysms
Introduction
Late presentation of aortic false aneurysm following repair of acute type A aortic dissection occurs in 10% to 24% of cases (1). False aneurysms are caused by partial or total dehiscence of the vascular graft from the aortic wall and could involve the proximal or distal anastomotic suture lines, or both (2). This condition is associated with high mortality if left untreated (1,2). False aneurysms may expand, rupture, compress, or erode into neighboring structures. The surgical treatment of this condition is complex, and involves considerable risk (2-4). Herein we describe a combination of surgical strategies that we believe may be used effectively to reduce the surgical risk of these complex operations.
Surgical technique
The operative steps can be summarized as follows:
Patient preparation
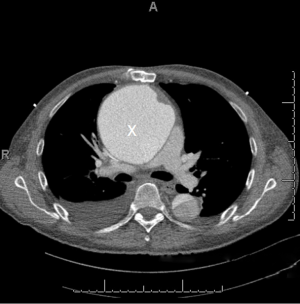
After induction of general anesthesia and intubation, patient monitoring is performed using Bispectral Index (BIS) (cerebral monitoring), central venous lines, Transesophageal Echocardiogram (TEE), right radial and left femoral arterial lines. In the presence of large false aneurysms (Figure 1), TEE insertion may be delayed until the chest is entered to avoid the risk of rupture caused by TEE probe manipulation.
Cannulation for CPB
The left common carotid artery is exposed via a small incision anterior to the left sternocleidomastoid muscle. The patient is fully heparinized. The artery is encircled with a tourniquet and then temporarily clamped proximally and distally. An 8 mm Hemashield graft is anastomosed in an end-to-side fashion using a running 5-0 polypropylene suture and is used for arterial cannulation. The right femoral vein is cannulated percutaneously using a long 29F (or 25F) single stage venous cannula placed under TEE guidance (if a TEE probe is used prior to chest re-entry).
Cardiopulmonary bypass (CPG) and left ventricular venting
CPG is initiated and maintained at 2.2 L/min/m2, cooling the patient to a nasopharyngeal temperature of 18 °C while monitoring BIS signals. While cooling, the patient is placed in Trendelenburg, a 2-inch left anterior minithoracotomy incision, is performed at the 6th intercostal space along the mid-clavicular line. The left ventricular apex is cannulated with a left ventricular vent to avoid distension in case of fibrillation or asystole. This is particularly useful in the presence of aortic valve insufficiency. The vent is kept at low suction while the heart is beating to avoid cavitation and air embolism, increasing suction as the heart fibrillations or arrests.
Chest re-entry
Once the target nasopharyngeal temperature is reached, low-flow antegrade cerebral perfusion is established at 0.5-1.0 L/min (18 °C) by snaring the proximal left carotid artery. Sternal wires are removed and sternal re-entry is performed using an oscillating saw and heavy scissors for the posterior sternal table. Heavy Rake retractors facilitate upward lifting of the sternum.
False aneurysm management
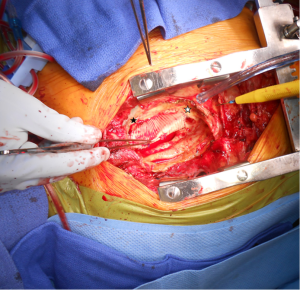
After the mediastinal entry, the false aneurysm is opened, and any fluid is removed and cultured. The integrity of the vascular graft and anastomotic suture lines are rapidly assessed for dehiscence (Figure 2). If a limited area of dehiscence is identified in the absence of evident infection, the graft maybe repaired directly or by using a patch. Otherwise the graft is replaced with another vascular graft or homograft. The graft is excised and the field irrigated with antibiotic solution. The aortic valve may be inspected at this stage, and in the presence of aortic insufficiency, aortic valve commissuroplasty, replacement, or root replacement may be considered.
Myocardial protection
Myocardial protection is provided by direct antegrade cardioplegia through the coronary ostia. If the vascular graft is salvaged, exposure of the coronary ostia may be achieved through a small opening in the proximal portion of the vascular graft. A retrograde catheter in the coronary sinus may be also inserted at this stage, although this may require further dissection of the right atrium, potentially a time-consuming maneuver in the presence of dense mediastinal adhesions.
Separation from CPB
De-airing maneuvers are performed, the left carotid artery snare is released, full CPB flow is restored, and rewarming is carried out. The left ventricular vent is removed and the left ventricular apical purse-strings are tied. CPB is turned off upon warming the patient. The venous cannula is removed and a skin purse-string suture is tied and pressure applied for 10-15 minutes. The 8 mm Hemashield graft is cut at its base using a vascular stapler and the stump reinforced with a running 5-0 prolene suture.
Management of the surgical incision
After confirming hemostasis, antibiotic solution is used to irrigate the mediastinum, the field is packed with laps, mediastinal and pleural drains are placed, and the chest is left open, covered by Esmarc barrier and Ioban draping. Postoperatively, the timing being dictated by the patient’s clinical progress, the plastic surgery team is consulted to proceed with unilateral or bilateral pectoralis major muscle flaps to cover the vascular graft. We believe this strategy is particularly useful in case of a mediastinal infection. Alternatively, omentum maybe used.
Discussion
False aneurysm formation is a rare complication of aortic surgery with high mortality (2). The incidence is higher in aortic dissection repair, probably as a result of greater friability of aortic tissues (2). Other risk factors include mediastinal infection, Takayasu’s arteritis, and Behcet’s disease (2). The time interval for developing a false aneurysm is variable and can be as far out as 17 years (2,5). For this reason, long term CT scan monitoring of the chest is recommended with a low threshold for even minor symptoms.
Key challenges when operating on patients with false aneurysm of the ascending aorta include chest re-entry, CPB cannulation, cerebral and myocardial protection, venting of the left ventricle, and closure of the incision. In this article we describe several useful strategies that we believe can be used in combination to lower the operative risks associated with these complex operations.
Although Mohammadi et al. (2) described bilateral carotid artery cannulation for CPB, we have consistently utilized isolated left carotid artery cannulation for complex aortic procedures. Left carotid artery and right femoral vein cannulation allows for rapid institution of CPB and cooling prior to sternal re-entry. This strategy avoids the hazards of femoral artery cannulation and retrograde aortic perfusion, which can be problematic in patients with aortic dissection extending to the thoracoabdominal aorta. This approach allows for selective antegrade cerebral perfusion once deep hypothermia is reached and results in retrograde blood flow in the right carotid and vertebral arteries, thereby reducing the risk of embolization to the brain.
A separate left mini-thoracotomy incision to vent the left ventricle prior to sternal re-entry, as is a useful adjunct especially in patients with aortic insufficiency to avoid left ventricular distension during cooling (6). Venting along with direct antegrade allows for adequate myocardial protection.
In our experience, delayed chest closure by using pectoralis or omental flaps is a useful strategy following false aneurysm repair as we believe it covers the vascular graft and fills the mediastinal space with vascularized tissue, thereby facilitating healing and possibly reducing the risk of recurrent infection.
In contrast to other papers describing the use of femoral cannulation for CPB and circulatory arrest without left ventricular venting, we believe the strategies described herein allow for cerebral and myocardial preservation (1). The surgical strategies described above have been reported previously in a variety of clinical settings. However, this article addresses their use in combination in patients with false aneurysm of the ascending aorta which appears advantageous in our experience to reduce the associated risks.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Erguneş K, Yilik L, Yurekli I, et al. Surgery for false aneurysm developing after type A acute aortic dissection. Asian Cardiovasc Thorac Ann 2014. [Epub ahead of print]. [PubMed]
- Mohammadi S, Bonnet N, Leprince P, et al. Reoperation for false aneurysm of the ascending aorta after its prosthetic replacement: surgical strategy. Ann Thorac Surg 2005;79:147-52; discussion 152. [PubMed]
- Razzouk A, Gundry S, Wang N, et al. Pseudoaneurysms of the aorta after cardiac surgery or chest trauma. Am Surg 1993;59:818-23. [PubMed]
- Sullivan KL, Steiner RM, Smullens SN, et al. Pseudoaneurysm of the ascending aorta following cardiac surgery. Chest 1988;93:138-43. [PubMed]
- Kobuch R, Hilker M, Rupprecht L, et al. Late reoperations after repaired acute type A aortic dissection. J Thorac Cardiovasc Surg 2012;144:300-7. [PubMed]
- Ito K, Yaku H, Shimada Y, et al. Left ventricular apex venting during deep hypothermia in a case of difficult re-entry into the mediastinum. J Cardiovasc Surg (Torino) 2001;42:493-4. [PubMed]


