
Autoantibodies detection in anti-N-methyl-D-aspartate receptor encephalitis
Introduction
Autoimmune encephalitis (AE) covers a group of central nervous system diseases with clinical manifestations as neurological and/or psychiatric symptoms. AE is severe but treatable. It has gained increasing attention currently. The body’s immune function can be disturbed under certain conditions such as tumor and infection, producing antibodies directed against neuronal autoantigens. Anti-neuronal antibodies include antibodies against cell surface, synaptic and intraneuronal antigens (1,2). Antibodies against cell surface antigens can directly influence the neurotransmission and excitability by targeting molecules including encephalitis: anti-N-methyl-D-aspartate (NMDA) receptor and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors via changing the function of the target protein (2,3). Antibodies may act as either agonist or antagonist on receptors (4), interfere with adjacent molecular interactions or reduce the expression of receptors on cell surface by altering the localization of membrane receptors or causing receptor internalization (i.e., anti-NMDAR antibodies) (5,6). Moreover, they can lead to the opening of transmembrane ion channels or cell death because of complement deposition and activation of natural killer cells. Antibodies against synaptic antigens are believed to alter the release or responsiveness of neurotransmitters (3). In contrast, antibodies against intraneuronal antigens (i.e., anti-Hu, anti-Yo and anti-Ma) are most likely not directly pathogenic, probably an epiphenomenon of T-cell-mediated immune response and classified as paraneoplastic neurological syndrome-related onconeural antibodies (7,8). Further discoveries showed that these antibodies caused cellular dysfunction or injury through multiple effector mechanisms. Intracellular antigens were not accessible to immune attack in situ; but upregulated major histocompatibility complex class I molecules in a pro-inflammatory cytokine milieu after proteasomal degradation, and then they were accessible to peptide-specific cytotoxic T cells (3).
Back in 2005, a case that the condition of one patient with paraneoplastic encephalitis was severe and potentially fatal, but the treatment-was effective (9). Two years later, Dalmau et al. used rat tissue, neuronal cultures, and human embryonic kidney 293 (HEK293) cells expressing subunits of the NMDAR to analyze serum/cerebrospinal fluid (CSF) antibodies (10). They discovered that the autoantigen expressed on the neuronal membrane was the NMDAR, and, for the first time, proposed the pathological role of anti-NMDAR antibody in this encephalitis in detail (10). Since then, many new antibodies associated with AE have been discovered, such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) antibodies (10), γ-aminobutyric acid type B receptor (GABABR) antibodies (11),antibodies against glutamic acid decarboxylase (GAD) (12) and leucine-rich, glioma-inactivated 1 (LGI1) (13).
Anti-NMDAR encephalitis is the most common and thoroughly studied AE (14). Anti-NMDAR encephalitis commonly presents with symptoms such as psychosis, epilepsy, dysfunction of the autonomic nervous system and various disturbances in movement (14). Although tumors such as teratoma were often found in patients with anti-NMDAR encephalitis, they were not the indispensable factor inducing disease, because a significant proportion of patients still did not have tumors (15). Early application of immunotherapy or tumor resection was effective, which depended on timely diagnosis (16). Anti-NMDAR encephalitis is distinguished by the presence of autoantibodies primarily against NMDAR subunit 1 (NR1) in CSF and/or serum (10). Clinically, a definitive diagnosis requires the detection of pathogenic anti-NMDAR antibodies. Some anti-NMDAR encephalitis patients could be misdiagnosed as mentally ill and missed early effective immunosuppressive therapy if they showed isolated psychiatric episodes in the early days. However, once these misdiagnosed patients received the individualized immunotherapy (first-line immunotherapy including corticosteroids, intravenous immunoglobulins or plasma exchange; or second-line immunotherapy such as rituximab or cyclophosphamide, or both, or long-term immunotherapy (mycophenolate mofetil or azathioprine >1 year),their symptoms could be gradually cured (17). Thus, improving the sensitivity and specificity of autoantibody diagnostic bioassay in NMDAR antibodies is meaningful. Clinicians generally make the diagnosis according to the standard proposed by Graus et al. in 2016 (18), which is dependent on clinical symptoms and the presence of anti-NMDAR antibodies in serum and CSF. A definite diagnosis can be confirmed when CSF anti-NMDAR antibodies are detected. However, on the contrary, some asymptomatic individuals were found with positive antibodies (19), which did not meet the diagnostic criteria.
This study aimed to provide an overview on detection assays of NMDAR antibodies, focusing on their clinical significance, sensitivity and specificity to increase the awareness for this previously under-recognized disease.
Antibody detection assays for AE
The clinical manifestations of AE are varied (14,20). Routine serological, CSF, electroencephalograms (21) and imaging examinations (22) are not conclusive to the diagnosis of anti-NMDAR encephalitis. If anti-NMDAR antibodies are detected in CSF, the diagnosis can be confirmed owing to its high specificity (15). Four different techniques are used to detect antibodies in anti-NMDAR encephalitis: tissue-based assay (TBA) on the brain tissue of rodents using indirect immunohistochemical (IHC) analysis or- indirect immunofluorescence, culture of dissociated hippocampal neurons from rats, and cell-based assay (CBA) with HEK293 cells (21,23).
At present, immunofluorescence has been widely used in laboratories. A positive group showed specific binding with fluorescent markers after incubation with anti-human immunoglobulin G (IgG) with fluorescent labeling. The steps and results of IHC analysis were similar to those of immunofluorescence (24). The IHC analysis could detect the presence of most antibodies, but lacks specificity.
Hippocampal neurons were cultured from embryonic rats as previously described (25). They were applied to patients with anti-NMDAR encephalitis to examine the effects of antibodies on neurons rather than to prove the presence of anti-NMDAR antibodies. The neurons were incubated with antibodies against NR1 followed by the appropriate fluorescence-conjugated secondary antibodies. Imaging and quantification were conducted to determine the amount of immunolabeling of NMDA receptors by patients’ antibodies (10,21). These antibodies decreased the number of NMDA-receptor clusters in postsynaptic dendrites selectively and reversibly. In addition, neurons incubated with IgG isolated from the serum of patients or control showed that the amount of patients’ IgG decreased the cell-surface fraction of NMDA receptors. The correlation coefficient between the patients’ antibody titers and the reduction in the number of receptors on the cell surface was positive (21).
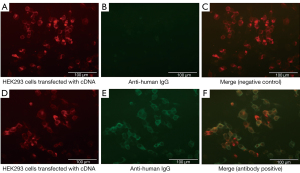
There different subunits (NR1–3) were involved in the formation of NMDAR as a heterotetramer. NR1, an essential subunit of a fully functional NMDAR on the cell surface, binds to glycine, while NR2 bind glutamate (26). The anti-NMDAR antibody belongs to the immunoglobulin G (IgG) subclass, it is directed against NR1 and NR2B subunits, but mainly binds to NR1 by amino acid 369 in the N-terminal epitope (15). In CBA, HEK293 cells transfected with NR1 complementary (c)DNA are used. IgG antibodies against the subunit NR1 of NMDAR are highly specific to anti-NMDAR encephalitis and have been demonstrated as an indicator of this disease (15,21). HEK293 cells could be transfected with plasmids expressing both NR1 and NR2B cDNA in a certain ratio to enhance the antigen-antibody reaction as much as possible (15,27). An enhanced green fluorescent protein expression vector was co-transfected with cells to visualize the existence of cDNAs. After transfection, the cells were incubated with patients’ serum or CSF with subsequent fixation using 4% paraformaldehyde (live CBA) (27). Alternatively, the cells were fixed before being incubated with sera or CSF (pre-fixed CBA) (28). A secondary antibody—anti-human IgG with fluorescent labeling could cause double fluorescent labeling and further reduce the possibility of false positives. The negative group showed no binding (Figure 1A-1C), while the positive group showed specific binding to fluorescent markers in CBA (Figure 1D-1F). The binding of antibodies to antigens on the cell surface or tissue are visualized using a fluorescence microscope to obtain a semi-quantitative result (29) and scored visually on a scale from 0 (no binding) to 4 (very strong binding). A score of 0–0.5 was considered as normal. Otherwise, the result was antibody positive (30). A low CSF antibody titer was considered as 1:1 or 1:3.2, while a high titer as 1:10 or 1:32 (31).
Application in clinical scenarios
Clinically, a CSF NMDAR antibody titer of 1:10 is generally considered positive. Pre-fixed CBA and IHC analysis were frequently used for antibody detection. The former focused on the antigen antibody reaction between antibodies in samples and fixed cells overexpressing NMDAR; the latter targeted antibodies against cell surface or synaptic proteins using frozen sections of a rat brain (21,32). The CBA could detect antibodies against neuronal surface antigens. Combined with TBA or IHC analysis, CBA could further confirm the regions of the brain where the autoimmune response occurred. Live CBA was reported to have lower sensitivity compared with pre-fixed CBA (15). Actually, live cells might be destroyed after incubation with high antibody titers, resulting in a lower antibody detection rate. However, whether it was fixed or live cells, false-positive or false-negative results were achieved without confirmatory tests (e.g., IHC) (15,33,34), suggesting suggested that multiple methods should be used to ensure the accuracy of antibody detection results. Besides, anti-NMDAR antibodies in patients’ serum and CSF could show reactivity with live neurons. The IHC analysis on cultured hippocampus also revealed the presence of anti-NMDAR antibodies (21). However, the distribution of NMDA receptors on the surface of cultured neurons was influenced by patients’ antibodies, leading to the false-negative results in the IHC analysis. Patients’ antibody mediated NMDA receptors endocytosis, which decreased the number of NMDA receptor on the surface of neurons (21).These methods usually took several days to obtain the experimental results. Thus, cultured neurons were rarely used for a routine inspection to prove the presence of antibodies but were used for basic researches focusing on the effects of antibodies on neurons (35). Nevertheless, Dalmau who discovered the anti-NMDAR antibody suggested that antibody tests should include three methods (IHC, CBA, and cultured neurons). If one’s serum antibody was weakly positive in TBA, verification using another assay or analyzing CSF by CBA was needed to avoid missed diagnosis and false positives. When using this three-stage methodology in patients with schizophrenia, no antibody-positive case was found. The positive result only in CBA was possibly false positive according to Dalmau’s standard (36,37). Subsequently, many improved versions of CBA with higher sensitivity and specificity were developed (27,32). The three-phase gold standard is rarely used for routine inspections at present. Whether it is too strict to produce a false-negative result remains unknown.
Autoantibody testing is primarily performed in patients suspected with AE. CBA is the most widely used method due to its high specificity. Since CSF obtained by lumbar puncture was traumatic, some medical institutions tended to test antibodies only in serum (27,28,38). When both CSF and serum were examined, about 15% of serum results were false positive (15,39,40); however, these patients seemed to be elderly with milder neurologic symptoms and a low rate of tumors (41). In addition, other studies reported that antibodies were found only in CSF in some patients with anti-NMDAR encephalitis (42-44). In the largest study including 412 patients (paired serum and CSF), no patient was found to have antibodies only in serum. The antibodies in CSF correlated better with this disease than those in serum (3,37). However, if the diagnosis is delayed or patients are treated with plasma exchange or intravenous immunoglobulin, antibodies may be detected only in CSF. NMDAR antibody testing is also applied in patients diagnosed with postpartum psychosis and first-episode psychosis (28,45). Some of these patients showed positive results and were identified as having anti-NMDAR encephalitis subsequently. NMDAR antibodies were reported to be detectable in Creutzfeldt-Jakob disease (CJD) (46), schizophrenia (30), or neurodegenerative diseases (47). In these cases, serum was tested using CBA and revealed anti-NMDAR antibodies, whereas CSF was not tested or was negative in anti-NMDAR antibodies. These findings were not reproduced in studies using CBA and IHC analysis in combination to detect antibodies in both serum and CSF (21,36,48,49). The serum antibody titer from a patient diagnosed with CJD was 1:80, but the patient failed to respond to immunotherapy and eventually died (46). Despite the high specificity of CBA, false positives should be taken into account, especially when testing with serum only. If the autoantibody was detectable only with IHC analysis but not with CBA, or the antibody was detectable in serum but not in CSF, the result might be considered as false positive. Under this condition, clinical symptoms and differential diagnoses play an important role.
On comparing CBA with IHC analysis, a higher antibody-positive rate and a greater antigen detection range were found in IHC. This result was not surprising because CBA focused on the specific antigen-antibody reaction while IHC analysis targeted antibodies against cell surface proteins or synaptic proteins throughout the rodent’s brain (21,32). Thus, CBA would present a higher specificity to autoantibodies, while positive results detected by IHC analysis could reveal the existence of antibodies other than known antibodies. Hence, IHC analysis helped confirm the presence of autoantibodies, while CBA with high specificity helped confirm the presence of specific antibodies. Clinically, some patients who were antibody negative in CBA but positive in IHC analysis shared common symptoms with AE but failed to find out a definite cause. These patients might be classified as having other kinds of antibodies associated with AE but not anti-NMDAR encephalitis, such as AMPAR, GAD and LGI1, which required further detection of related antibodies. Despite failing to find the etiology in unexplained AE, positive results in IHC analysis still provided some reference for clinicians to make decisions. In anti-NMDAR encephalitis, the concrete epidemiology and optimal immunotherapy remains to be determined. However, early diagnosis and early treatment can bring better efficacy (21,50). Hence, it is extremely important to get a timely and correct diagnosis. Patients with delayed diagnosis, long course of illness, or persistent symptoms may have negative serum antibodies, but CSF titers may continue to rise (51). Combining CBA and IHC analysis to test both serum and CSF could increase the “positive rate” (for autoimmune cause), and also lower the rate of missed diagnosis. However, the evidence for treatment based on IHC-positivity is lacking.
Optimization of detection assays in practice
Many factors account for the inconsistent positivity of antibody detection between different laboratories, such as the process of preparing HEK 293 cells and slices of the brain, artificial visual judgment of the results and even different equipment in the laboratory. Multiple antibodies can be found in some laboratory tests; we should repeatedly verify to prevent false positives. On the contrary, NMDAR antibodies can also be found in some patients with herpes simplex encephalitis (HSE), a transient and subclinical synthesis of neuronal antibodies occurs, which becomes undetectable several months after the infection (52). Only a longer follow-up of patients HSE can clarify whether they have a propensity to develop AE. Some researchers try to improve and optimize the detection methods to improve the detection rate of positive samples.
The evaluation of cell surface staining by fluorescence microscopy is strongly dependent on the experience of investigators in CBA. Hence, Melanie Ramberger and his colleagues intended to find out whether flow cytometry [fluorescence-activated cell sorting (FACS)]—based assay was more objective and reliable to prove the presence of antibodies (53). FACS is a type of flow cytometry, cells need to stay alive; FACS was compared with live CBA. Human NMDAR antibodies were transfected into HEK293A cells to bind to the antibodies in serum, and the hCD2-EmGFP fusion protein was transfected to determine unspecific binding of serum antibodies. After transfection, the cells were detached by trypsin from tissue culture test plates and incubated with patients’ serum. Bound serum antibodies were detected by the allophycocyanin-conjugated AffiniPure goat anti-human IgG antibody. Washing buffer containing 7-amino-actinomycin D was used for incubation to exclude dead cells. The analysis was performed on a flow cytometer. The results showed that the specificity of FACS based assay was as high as that of CBA. However, FACS based assay had a lower sensitivity and high inter-assay variation. The high inter-assay variation might be due to the reason that not all samples were analyzed with the same batch of transfected and trypsinized cells. Hence, CBA is still a more reliable detection method.
In addition, single nanoparticle imaging in hippocampal neurons was reported to detect patients with low antibody titers, implying its high sensitivity. Single nanoparticle imaging relied on the binding of individual antibodies to their target and was therefore independent of the sample titer, contrary to other methods dependent on a sufficient amount of antibodies to provide a detectable signal. It is worth noting that unspecific trajectories were rarely detected without anti-NMDAR IgG (45). In this method, sensitivity may be higher than that of CBA in a larger- sample study (26). Single nanoparticle imaging in hippocampal neurons would play an important role in clinical diagnosis because of its high sensitivity and specificity, however, it is technically demanding and time consuming.
Chiu and his colleges found that replacing reporter fluorophore fluorescein isothiocyanate in the commercial kits with Alexa Fluor 488 could obtain better detection result, especially in samples with low titers (17) because the fluorescence from Alexa Fluor 488 was brighter and more stable (54,55). A more sophisticated optic/imaging device is recommended because it allows even weak signals to be displayed from samples with low titers. A double labeling approach, widely used at present, with a mouse anti-NR1 mAb and biotinylated goat anti-human IgG could avoid false-positive results.
Clinical implications for antibody testing
As NMDAR antibodies target neuronal receptors and weaken the glutamatergic transmission, mental and cognitive impairment manifestations are apparent in patients with anti-NMDAR encephalitis. Some patients with anti-NMDAR encephalitis were misdiagnosed with psychosis because only neuropsychiatric symptoms were observed in the early stage (45). In contrast, patients with anti NMDAR encephalitis with chronic mental illness usually did not develop anti-NMDAR encephalitis (37,39). Additionally, overlapping autoimmune antibodies were found in some patients (40). Thus, solving these diagnostic challenges is more complex. The treatment of anti-NMDAR encephalitis focuses on improving prominent psychiatric symptoms and executive dysfunction. First-line therapies were effective with good outcomes, although some patients even required intensive care (14,56,57). Hence, patients showing first-episode psychosis or neuropsychiatric symptoms chiefly orofacial dyskinesias and/or autonomic dysfunction should be screened for NMDAR antibodies to help them get an accurate diagnosis and receive appropriate treatment.
For both CBA and TBA to detect antibodies, clinical symptoms are more important in diagnosing anti-NMDAR encephalitis. One patient with a serum antibody titer of 1:320 could not be diagnosed with anti-NMDAR encephalitis, because CSF antibody was not found and his clinical symptoms did not support the diagnosis of anti-NMDAR encephalitis (58). If one patient is positive for serum NMDAR antibodies, is negative for CSF, and has no neuropsychiatric symptoms, this patient cannot be diagnosed with anti-NMDAR encephalitis. Or, in some patients with multiple core symptoms, IHC analysis reveals unknown antibodies while the CBA for an NMDAR antibody is negative. Immunosuppression treatment may be effective for those showing multiple core symptoms without an NMDAR antibody; however, reliable evidence is lacking.
Besides, HSE caused by herpes simplex virus (HSV) infection is most closely related to anti-NMDAR encephalitis. Virus infection causes the destruction of neurons. Then, the exposure of neuronal surface antigen and the breaking of immune tolerance trigger the autoimmune response. It is also possible that B cell activation (nonspecific) or molecular mimicry is involved. This is because HSV and NMDAR share the same antigen epitopes. Therefore, it is speculated that viral infection causes B cell activation and production of antibodies against the virus, which cross-reacts with NMDAR, leading to the occurrence of anti-NMDAR encephalitis (59). Recent studies demonstrated that 7% of patients with HSE were positive for IgG NMDAR antibodies (60). Moreover, a child with post-HSE choreoathetosis was found to have NMDAR antibodies, who did not improve with antiviral therapy but recovered after immunotherapy. The findings indicated that a subgroup of post-HSE represented a separate disease entity, which actually was anti-NMDAR encephalitis. Patients with relapsing HSE or prolonged atypical symptoms should be tested for NMDAR IgG antibodies in CSF and serum when they have negative CSF polymerase chain reaction for HSV. Combining CBA and IHC analysis to screen suspected AE can reduce the rate of missed diagnosis and pick out more potential patients.
Modified Rankin score (mRS) was used to access patients’ disease severity and outcomes in the acute phase and follow-up period. Anti-NMDAR antibody titers correlated with mRS scores and clinical improvement (27).Patients with good prognosis had significantly lower anti-NMDAR antibody levels and mRS scores, whereas patients with unimproved symptoms or poor outcomes and deaths had high antibody levels, although they received immunotherapy. Whatever the outcomes, antibody titers declined over time (15,21). Hence, spontaneous clinical improvement and antibody disappearance in some cases were probably due to slow spontaneous regression of the immune response (61). Additionally, immunotherapy led to a decline in antibody titers. Patients with better outcomes had declining levels of CSF antibodies early in the disease, while patients with clinical recurrence and deterioration showed increased CSF antibody titers. This correlation was not obvious in serum titers. Dalmau and colleagues found that the change in NMDAR antibody concentrations in CSF was more closely related to clinical relapses compared with those in serum (15). To note, the treatment (plasma exchange or intravenous immunoglobulin) could temporarily decreased the levels of antibodies in serum but not in CSF (62). The difference in titers between patients with poor prognosis and those with good prognosis might be greater than statistical results suggested, as more intensive immunotherapy might be administered to those with poor outcomes (15). However, Irani and his colleagues found that though the intrathecal synthesis of anti-NMDAR antibody was obvious, the absolute level of antibody in serum was 13.5 times higher than that in CSF on average, although the intrathecal synthesis of the anti-NMDAR antibody was obvious (27), serum antibody titers were also considered to be correlated with the improvement of clinical symptoms (43). The heterogeneity of testing methods between different laboratories might account for the inconsistent conclusion. Besides, each CBA had its specificity in terms of techniques used, such as fixed or live cell, transfecting with different NR subunit plasmids, and ratios. Also, the NMDAR conformation in the rodent brain or HEK cells might not be the same as that in humans.
Relapse means the occurrence of new symptoms or the aggravation of original symptoms after more than 2 months of the convalescence period. Patients with undetected or recurrent tumors (21) or non-paraneoplastic cases as well as those did not receive timely immunotherapy were more likely to relapse (63). Clinical recovery does not mean the disappearance of serum and CSF antibodies, some patients could still show positive antibodies in serum and CSF (15,64,65). Hence, clinical decisions should be based on a combination of symptoms, antibody detection results and other auxiliary examination (18). These patients are supposed to attend the immunological follow-up and tumor screening (14). The determination of baseline serum and CSF titers after recovery is potentially helpful in classifying new-onset symptoms as possible relapses, predicting disease risk, and managing the disease (according to the rise in the titer) (15). Early immunotherapy and tumors removal could effectively improve outcomes, reduce antibody levels and prevent relapses (27).
In China, the earliest case diagnosed with anti-NMDAR encephalitis was in 2010. Subsequently, more centers gradually started clinical research on anti-NMDAR encephalitis. The clinical research scope covered biomarkers, genetics, and antibody testing, but only a limited number of studies focused on the detection assay of AE in China. For example, Liu and his colleagues used TBA to detect antibodies in CSF in 739 patients. Of these, 37 patients had a neuronal antibody pattern. In another study, 17 patients were detected with NMDAR antibodies. The conclusion of this study was similar to that of another previous study (34). These results showed the advantages of TBA in discovering the AE of unknown antigen, especially in CSF. Commercially available CBA is frequently used for anti-NMDAR antibody detection in China. The results also showed that the positive rate of CSF was higher than that of serum (66,67). However, the shortcoming lay in the small sample size of these studies.
Other aspects, such as clinical characteristics, magnetic resonance imaging (MRI), immunotherapy regimens and long-term outcomes of patients with anti-NMDAR encephalitis in China have also been described. These clinical researches in the Chinese population revealed that anti-NMDAR encephalitis had unpredictable MRI findings that easily obscured its diagnosis and caused serious sequelae (68). An NMDAR antibody test is required for a timely diagnosis and immunotherapy (16). Most patients with severe anti-NMDAR encephalitis would eventually achieve good long-term prognoses after receiving early, positive, and unremitting combined immunotherapy and life support (69). Significantly, the largest Chinese anti-NMDAR encephalitis cohort so far, which recruited 220 patients, showed that the primary clinical presentations were psychosis and seizures (70). Tumors were not frequent, the incidence of ovarian teratoma in women was the maximum, and only one man had lung cancer. Most patients (99.5%) received first-line therapy, and only 7.3% received second-line immunotherapy. More than half of the patients were administered long-term immunotherapy. During the first year of follow-up, a large majority of patients (94.1%) achieved a good outcome, 2.3% of patients died, and 17.3% experienced relapses. First-line immunotherapy is effective in managing anti-NMDAR encephalitis in the acute phase. Although relapse is relatively common, most patients reached favorable outcomes with combined first-line and long-term immunotherapy (70).
Conclusions
AE is a treatable immune-mediated disorder that it can be diagnosed by the specific autoantibody detection. The diagnosis of AE should be based on antibody testing combined with clinical symptoms. IHC analysis involves could pick out more potential patients than CBA. Antibodies should be detected in both serum and CSF using IHC analysis and CBA to avoid misdiagnosis. CSF antibody titers are believed to be more clinically relevant in anti-NMDAR encephalitis. However, prospective studies are needed to determine the prognostic value of antibody titers because some clinically cured patients still have detectable NMDAR antibodies detectable. A long-term follow-up is recommended. Further studies should pay attention to potential biomarkers in serum for diagnosis or assessment of this disease because CSF testing is invasive and not always available.
Acknowledgments
Funding: This work was funded by the National Natural Science Foundation of China (No. 81673950), Guangdong Provincial Science and Technology Plan Projects (No. 2017A020215182), and Natural Science Foundation of Guangdong Province (No. 2019A1515011434).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hai-Feng Li and Xiangjun Chen) for the series “Laboratory Investigations in Neuroimmunological Diseases and Their Clinical Significance” published in Annals of Translational Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-20-2279/coif). The series “Laboratory Investigations in Neuroimmunological Diseases and Their Clinical Significance” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lancaster E, Dalmau J. Neuronal autoantigens--pathogenesis, associated disorders and antibody testing. Nat Rev Neurol 2012;8:380-90. [Crossref] [PubMed]
- van Coevorden-Hameete MH, de Graaff E, Titulaer MJ, et al. Molecular and cellular mechanisms underlying anti-neuronal antibody mediated disorders of the central nervous system. Autoimmun Rev 2014;13:299-312. [Crossref] [PubMed]
- Iorio R, Lennon VA. Neural antigen-specific autoimmune disorders. Immunol Rev 2012;248:104-21. [Crossref] [PubMed]
- Sillevis Smitt P, Kinoshita A, De Leeuw B, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med 2000;342:21-7. [Crossref] [PubMed]
- Mikasova L, De Rossi P, Bouchet D, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain 2012;135:1606-21. [Crossref] [PubMed]
- Boyse EA, Stockert E, Old LJ. Modification of the antigenic structure of the cell membrane by thymus-leukemia (TL) antibody. Proc Natl Acad Sci U S A 1967;58:954-7. [Crossref] [PubMed]
- Albert ML, Austin LM, Darnell RB. Detection and treatment of activated T cells in the cerebrospinal fluid of patients with paraneoplastic cerebellar degeneration. Ann Neurol 2000;47:9-17. [Crossref] [PubMed]
- Roberts WK, Deluca IJ, Thomas A, et al. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8+ T cells. J Clin Invest 2009;119:2042-51. [Crossref] [PubMed]
- Vitaliani R, Mason W, Ances B, et al. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol 2005;58:594-604. [Crossref] [PubMed]
- Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25-36. [Crossref] [PubMed]
- Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009;65:424-34. [Crossref] [PubMed]
- Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010;9:67-76. [Crossref] [PubMed]
- Boronat A, Sabater L, Saiz A, et al. GABA(B) receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology 2011;76:795-800. [Crossref] [PubMed]
- Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010;9:776-85. [Crossref] [PubMed]
- Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011;10:63-74. [Crossref] [PubMed]
- Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 2014;13:167-77. [Crossref] [PubMed]
- Huang X, Fan C, Wu J, et al. Clinical analysis on anti-N-methyl-D-aspartate receptor encephalitis cases: Chinese experience. Int J Clin Exp Med 2015;8:18927-35. [PubMed]
- Chiu NC, Lin YJ, Tzang RF, et al. Optimization of an Anti-NMDA Receptor Autoantibody Diagnostic Bioassay. Front Neurol 2018;9:661. [Crossref] [PubMed]
- Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391-404. [Crossref] [PubMed]
- Armangue T, Titulaer MJ, Sabater L, et al. A novel treatment-responsive encephalitis with frequent opsoclonus and teratoma. Ann Neurol 2014;75:435-41. [Crossref] [PubMed]
- Guan HZ, Ren HT, Cui LY. Autoimmune Encephalitis: An Expanding Frontier of Neuroimmunology. Chin Med J (Engl) 2016;129:1122-7. [Crossref] [PubMed]
- Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091-8. [Crossref] [PubMed]
- Gastaldi M, Waters P, Vincent A. Detection of NMDARs Antibodies in Encephalitis. Methods Mol Biol 2017;1677:117-26. [Crossref] [PubMed]
- Chen QM, Qu HD, Qian WD, et al. Correlation between neuronal antibodies and limbic encephalitis in Chinese Han subjects. Genet Mol Res 2015;14:2312-21. [Crossref] [PubMed]
- Elmariah SB, Oh EJ, Hughes EG, et al. Astrocytes regulate inhibitory synapse formation via Trk-mediated modulation of postsynaptic GABAA receptors. J Neurosci 2005;25:3638-50. [Crossref] [PubMed]
- Lynch DR, Anegawa NJ, Verdoorn T, et al. N-methyl-D-aspartate receptors: different subunit requirements for binding of glutamate antagonists, glycine antagonists, and channel-blocking agents. Mol Pharmacol 1994;45:540-5. [PubMed]
- Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010;133:1655-67. [Crossref] [PubMed]
- Bergink V, Armangue T, Titulaer MJ, et al. Autoimmune Encephalitis in Postpartum Psychosis. Am J Psychiatry 2015;172:901-8. [Crossref] [PubMed]
- Leite MI, Jacob S, Viegas S, et al. IgG1 antibodies to acetylcholine receptors in 'seronegative' myasthenia gravis. Brain 2008;131:1940-52. [Crossref] [PubMed]
- Zandi MS, Irani SR, Lang B, et al. Disease-relevant autoantibodies in first episode schizophrenia. J Neurol 2011;258:686-8. [Crossref] [PubMed]
- Miao A, Du M, Wang L, et al. Analysis of Relation Between Electroclinical Features and Cerebrospinal Fluid Antibody Titers in Patients With anti-NMDAR Encephalitis. Clin EEG Neurosci 2019;50:56-62. [Crossref] [PubMed]
- Wandinger KP, Saschenbrecker S, Stoecker W, et al. Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis. J Neuroimmunol 2011;231:86-91. [Crossref] [PubMed]
- Hara M, Martinez-Hernandez E, Ariño H, et al. Clinical and pathogenic significance of IgG, IgA, and IgM antibodies against the NMDA receptor. Neurology 2018;90:e1386-94. [Crossref] [PubMed]
- Zandi MS, Paterson RW, Ellul MA, et al. Clinical relevance of serum antibodies to extracellular N-methyl-D-aspartate receptor epitopes. J Neurol Neurosurg Psychiatry 2015;86:708-13. [Crossref] [PubMed]
- Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 2010;30:5866-75. [Crossref] [PubMed]
- Masdeu JC, González-Pinto A, Matute C, et al. Serum IgG antibodies against the NR1 subunit of the NMDA receptor not detected in schizophrenia. Am J Psychiatry 2012;169:1120-1. [Crossref] [PubMed]
- Pollak TA, McCormack R, Peakman M, et al. Prevalence of anti-N-methyl-D-aspartate (NMDA) receptor corrected antibodies in patients with schizophrenia and related psychoses: a systematic review and meta-analysis. Psychol Med 2014;44:2475-87. [Crossref] [PubMed]
- Davies G, Irani SR, Coltart C, et al. Anti-N-methyl-D-aspartate receptor antibodies: a potentially treatable cause of encephalitis in the intensive care unit. Crit Care Med 2010;38:679-82. [Crossref] [PubMed]
- Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157-65. [Crossref] [PubMed]
- Titulaer MJ, Höftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti–N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2014;75:411-28. [Crossref] [PubMed]
- Guasp M, Módena Y, Armangue T, et al. Clinical features of seronegative, but CSF antibody-positive, anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm 2020;7:e659. [Crossref] [PubMed]
- Punja M, Pomerleau AC, Devlin JJ, et al. Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis: an etiology worth considering in the differential diagnosis of delirium. Clin Toxicol (Phila) 2013;51:794-7. [Crossref] [PubMed]
- Suh-Lailam BB, Haven TR, Copple SS, et al. Anti-NMDA-receptor antibody encephalitis: performance evaluation and laboratory experience with the anti-NMDA-receptor IgG assay. Clin Chim Acta 2013;421:1-6. [Crossref] [PubMed]
- Greiner H, Leach JL, Lee KH, et al. Anti-NMDA receptor encephalitis presenting with imaging findings and clinical features mimicking Rasmussen syndrome. Seizure 2011;20:266-70. [Crossref] [PubMed]
- Jézéquel J, Rogemond V, Pollak T, et al. Cell- and Single Molecule-Based Methods to Detect Anti-N-Methyl-D-Aspartate Receptor Autoantibodies in Patients With First-Episode Psychosis From the OPTiMiSE Project. Biol Psychiatry 2017;82:766-72. [Crossref] [PubMed]
- Mackay G, Ahmad K, Stone J, et al. NMDA receptor autoantibodies in sporadic Creutzfeldt-Jakob disease. J Neurol 2012;259:1979-81. [Crossref] [PubMed]
- Deakin J, Lennox BR, Zandi MS. Antibodies to the N-methyl-D-aspartate receptor and other synaptic proteins in psychosis. Biol Psychiatry 2014;75:284-91. [Crossref] [PubMed]
- Grant I, Atkinson JH, Gouaux B. Research on medical marijuana. Am J Psychiatry 2012;169:1119-20; author reply 1120. [Crossref] [PubMed]
- Grau-Rivera O, Sánchez-Valle R, Saiz A, et al. Determination of neuronal antibodies in suspected and definite Creutzfeldt-Jakob disease. JAMA Neurol 2014;71:74-8. [Crossref] [PubMed]
- Seki M, Suzuki S, Iizuka T, et al. Neurological response to early removal of ovarian teratoma in anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry 2008;79:324-6. [Crossref] [PubMed]
- Miya K, Takahashi Y, Mori H. Anti-NMDAR autoimmune encephalitis. Brain Dev 2014;36:645-52. [Crossref] [PubMed]
- Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol 2018;17:760-72. [Crossref] [PubMed]
- Ramberger M, Peschl P, Schanda K, et al. Comparison of diagnostic accuracy of microscopy and flow cytometry in evaluating N-methyl-D-aspartate receptor antibodies in serum using a live cell-based assay. PLoS One 2015;10:e0122037. [Crossref] [PubMed]
- Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, et al. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J Histochem Cytochem 1999;47:1179-88. [Crossref] [PubMed]
- Mahmoudian J, Hadavi R, Jeddi-Tehrani M, et al. Comparison of the Photobleaching and Photostability Traits of Alexa Fluor 568- and Fluorescein Isothiocyanate- conjugated Antibody. Cell J 2011;13:169-72. [PubMed]
- Finke C, Kopp UA, Prüss H, et al. Cognitive deficits following anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry 2012;83:195-8. [Crossref] [PubMed]
- Kayser MS, Titulaer MJ, Gresa-Arribas N, et al. Frequency and characteristics of isolated psychiatric episodes in anti–N-methyl-d-aspartate receptor encephalitis. JAMA Neurol 2013;70:1133-9. [Crossref] [PubMed]
- Mantere O, Saarela M, Kieseppä T, et al. Anti-neuronal anti-bodies in patients with early psychosis. Schizophr Res 2018;192:404-7. [Crossref] [PubMed]
- Nosadini M, Mohammad SS, Corazza F, et al. Herpes simplex virus-induced anti-N-methyl-d-aspartate receptor encephalitis: a systematic literature review with analysis of 43 cases. Dev Med Child Neurol 2017;59:796-805. [Crossref] [PubMed]
- Prüss H, Finke C, Höltje M, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 2012;72:902-11. [Crossref] [PubMed]
- Iizuka T, Sakai F, Ide T, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology 2008;70:504-11. [Crossref] [PubMed]
- Furneaux HF, Reich L, Posner JB. Autoantibody synthesis in the central nervous system of patients with paraneoplastic syndromes. Neurology 1990;40:1085-91. [Crossref] [PubMed]
- Gabilondo I, Saiz A, Galán L, et al. Analysis of relapses in anti-NMDAR encephalitis. Neurology 2011;77:996-9. [Crossref] [PubMed]
- Hansen HC, Klingbeil C, Dalmau J, et al. Persistent intrathecal antibody synthesis 15 years after recovering from anti-N-methyl-D-aspartate receptor encephalitis. JAMA Neurol 2013;70:117-9. [Crossref] [PubMed]
- Alexopoulos H, Kosmidis ML, Dalmau J, et al. Paraneoplastic anti-NMDAR encephalitis: long term follow-up reveals persistent serum antibodies. J Neurol 2011;258:1568-70. [Crossref] [PubMed]
- Wang XH, Fang F, Ding CH, et al. Anti-N-methyl-D-aspartate receptor encephalitis in seven children. Zhonghua Er Ke Za Zhi 2012;50:885-9. [PubMed]
- Wu Y, Shi Y, Zhang M, et al. Anti-N-methyl-D-aspartate receptor encephalitis:clinical analysis of 11 cases. Zhonghua Yi Xue Za Zhi 2015;95:2857-60. [PubMed]
- Zhang W, Cui L, Wang W, et al. Early identification of anti-NMDA receptor encephalitis presenting cerebral lesions in unconventional locations on magnetic resonance imaging. J Neuroimmunol 2018;320:101-6. [Crossref] [PubMed]
- Zhang Y, Liu G, Jiang M, et al. Clinical Characteristics and Prognosis of Severe Anti-N-methyl-D-aspartate Receptor Encephalitis Patients. Neurocrit Care 2018;29:264-72. [Crossref] [PubMed]
- Xu X, Lu Q, Huang Y, et al. Anti-NMDAR encephalitis: A single-center, longitudinal study in China. Neurol Neuroimmunol Neuroinflamm 2020;7:e633. [Crossref] [PubMed]


