
Predicted outcomes of subdividing M1-stage metastatic lung cancer based on the prognosis and the response to local consolidative therapy
Introduction
Lung cancer is the most common cancer worldwide and is also one of the leading causes of cancer-related mortality (1,2). Non-small cell lung cancer (NSCLC) accounts for more than 80% of all lung cancer cases (3,4). Appropriate and accurate therapy impacts morbidity and mortality outcomes. Determination of the therapeutic schedule for NSCLC depends on various factors, including but not limited to the period of disease, patient comorbidities or performance scores, and the biological features of carcinoma (3,5). Resection is the standard treatment for patients with an early-stage disease and medically suitable conditions (6,7), whereas multimodality therapy involving chemotherapy, radiotherapy, and surgical resection is recommended for locally advanced tumors (8,9). For patients with stage IV disease, systemic therapy, including chemotherapy, immune therapy, and targeted therapy, is the main strategy, and local consolidative therapy tends to be performed for patients with oligometastases (10-13).
The anatomic extent of malignant carcinoma is presented according to the TNM staging system. Seventy-six types of malignant tumors were described by TNM staging, and approximately 20 types of carcinoma, such as lung and prostate cancers, include further subdivisions of the M1 category (10,14,15). According to the American Joint Committee on Cancer (AJCC) 8th edition, the M1 stage of lung cancer has been divided into three subcategories: M1a, separate tumor nodule(s) in the contralateral lobe, a tumor with pleural or pericardial nodules, malignant pleural nodules, or pericardial effusion; M1b, single extrathoracic metastasis in a single organ; and M1c, multiple extrathoracic metastases in one or several organs (16). However, patients with stage IV NSCLC are thought to have the same prognosis in defiance of histological grade, EGFR mutation, PD-1 expression, and PD-L1 expression (17,18). Therefore, further subdivision of stage IV tumors is clinically important for predicting prognosis and guiding individualized treatment.
By performing a population-based study, we aimed to evaluate the prognostic effects of local consolidative therapy for patients with stage IV NSCLC and divide patients with stage IV NSCLC into different subcategories to stratify the prognoses. We present the following article in accordance with the TRIPOD reporting checklist (available at https://dx.doi.org/10.21037/atm-21-1383).
Methods
Patients and methods
Patients were selected from the SEER database, which includes clinical records for cancer occurrences in 18 areas of the United States corresponding to approximately 27.8% of the population (19). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). SEER*Stat Database: Incidence, SEER 18 Regs Research Data (with chemotherapy recode), Nov 2015 Sub (2000–2013) software (version 8.3.5; seer.cancer.gov/seerstat) was used to identify patients with NSCLC diagnosed from 2010 through 2013. Patients with lung cancer were selected using ICD-O-3 codes (C34.0–C34.9). SEER database includes information on patient demographics, the position and morphology of a primary carcinoma, the stage of a tumor at diagnosis, the first treatment modality, and survival status. Only patients who were diagnosed with stage IV NSCLC at the initial diagnosis and had only one malignant primary in lifetime were included. Figure 1 details the selection process for the inclusion of patients. All patients were at least 18 years old. Patients with tumors that were stage I to III, unknown follow-ups and basic information, or unknown bone involvement, brain involvement, liver involvement, or lung involvement and patients diagnosed by autopsy or death certificate were not included in the study. As a result, we selected a total of 30,583 patients in our cohort for analysis.
Distinguishing subgroups
To supplement the current M1 subdivision, individuals were divided into subgroups according to M1 stage and the presence or absence of liver involvement, which was identified as the most important prognostic factor in multivariable analysis (20). Patients in group A were diagnosed with M1c NSCLC with liver involvement; patients in group B were diagnosed with M1c NSCLC without liver involvement; patients in group C were diagnosed with M1b NSCLC with liver involvement; patients in group D were diagnosed with M1b NSCLC without liver involvement; and patients in group E were diagnosed with M1a NSCLC. And the null hypothesis is that the groups divided by the current M1 subcategory and involvement of liver have no significant difference in prognoses (P>0.05).
Statistical analysis
Cancer-specific mortality (CSM) was defined as death due to lung cancer utilizing the specific codes from the SEER database as in prior articles (21,22). Kaplan-Meier and log-rank tests were conducted to evaluate CSM rates. Pearson’s χ2 test and Fisher’s exact test were applied to analyze categorical data, while Student’s t-test and the Mann-Whitney U-test were applied to analyze numerical data. SPSS 23.0 software (IBM Corp., Armonk, New York, USA) was used to perform the statistical analysis. A Fine-Gray competing risk model was applied in multivariable analyses by using variables with P<0.05 in the univariable analysis. All analyses were double-tailed. A P value <0.05 was considered statistically significant.
Results
Baseline characteristics
Among the 172,884 patients diagnosed with lung cancer, 30,583 with stage IV NSCLC were identified, including 7,520 patients in the M1a group, 14,517 patients in the M1b group and 8,546 patients were in the M1c group (Figure 1). As shown in Table 1, the most common involved organs were the lung, bone, brain, and liver. Totally, 1,328 patients were lost to follow-up and censored. The median follow-up of the whole cohort was calculated to be 23.0 [95% confidence interval (CI), 22.4–23.6] months.
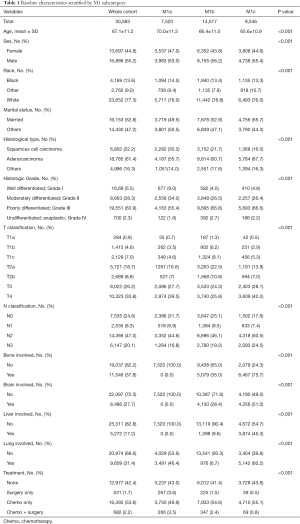
Full table
Association between clinicopathological characteristics of patients and CSM rates
The results of the univariable analysis showed that brain, liver, and bone involvement, male sex, adenocarcinoma, other marital statuses and advanced histological grade were associated with increased CSM rates. Using a competing risk regression model, advanced histological grade [Grade II vs. Grade I: subdistribution hazard ratio (SHR), 1.29; 95% CI, 1.17–1.43, P<0.001; Grade III vs. Grade I: SHR, 1.63; 95% CI, 1.48–1.80, P<0.001; Grade IV vs. Grade I: SHR, 1.77; 95% CI, 1.52 – 2.07, P<0.001], male sex (SHR, 1.21; 95% CI, 1.17–1.24; P<0.001), bone involvement (SHR, 1.28; 95% CI, 1.24–1.32; P<0.001), brain involvement (SHR, 1.23; 95% CI, 1.19–1.28; P<0.001), and liver involvement (SHR, 1.47; 95% CI, 1.41–1.53; P<0.001) were independent prognostic factors and other independent prognostic factors were shown in Table 2.
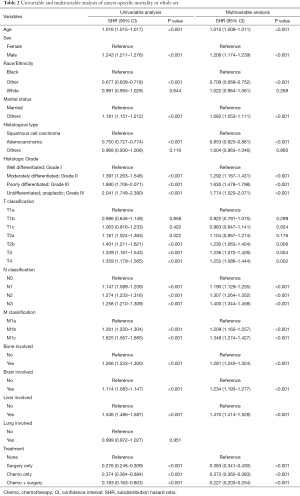
Full table
Subdivision of the M1 category
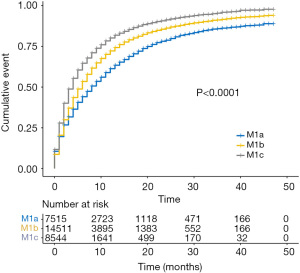
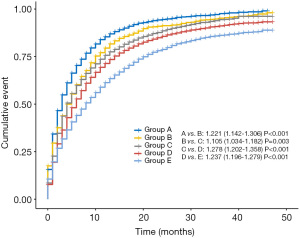
Patients with stage IV NSCLC were subdivided into three categories: M1a, M1b, and M1c. The CSM rates increased across the M1 subcategories (Figure 2). Compared with bone or brain involved, liver involved was an more important prognostic factor with a higher SHR value of 1.47. To create a concise model that is convenient for clinical practice, all the patients were grouped into five groups considering these two important factors: M1 subcategory and liver involvement: Group A, M1c NSCLC with liver involvement; group B, M1c NSCLC without liver involvement; group C, M1b NSCLC with liver involvement; Group D, M1b NSCLC without liver involvement; and group E, M1a NSCLC. The univariable analysis demonstrated the results that liver involvement was related to increased CSM rates in both M1b and M1c patients [A vs. B (B as the reference): SHR, 1.36; 95% CI, 1.30–1.43; P<0.001; C vs. D (D as the reference): SHR, 1.27; 95% CI, 1.20–1.35; P<0.001] (Figure 3).
Associations between treatment modalities and M1 subcategories with survival status
As shown in Figure 4, Kaplan-Meier curves showed reduced CSM rates after primary tumor surgery, chemotherapy, or combined therapy in patients with M1a, M1b, and M1c diseases. When monotherapy was compared with no therapy, primary tumor surgery was identified as a favorable prognostic factor, particularly for M1a cases (M1a: SHR, 0.35; 95% CI, 0.29–0.42; P<0.001; M1b: SHR, 0.43; 95% CI, 0.36–0.50; P<0.001; M1c: SHR, 0.57; 95% CI, 0.40–0.70; P<0.001) (Table 3). M1a patients benefited less from chemotherapy only than M1b or M1c patients (M1a: SHR, 0.41; 95% CI, 0.39–0.44; P<0.001; M1b: SHR, 0.37; 95% CI, 0.36–0.39; P<0.001; and M1c: SHR, 0.34; 95% CI, 0.32–0.36; P<0.001) (Table 3). Specifically, no significant difference in CSM rates was observed between patients treated with surgery only and those treated with chemotherapy only, with the exception of patients with M1a disease, who showed a worse prognosis when receiving only chemotherapy (SHR, 1.18; 95% CI, 0.98–1.43; P=0.085) (Table 3). Patients receiving combination therapy displayed the lowest SHR in M1a, M1b, and M1c groups (M1a: SHR, 0.27; 95% CI, 0.23–0.33; P<0.001; M1b: SHR, 0.20; 95% CI, 0.17–0.23, P<0.001; M1c: SHR, 0.27; 95% CI, 0.20–0.36; P<0.001) (Table 3).
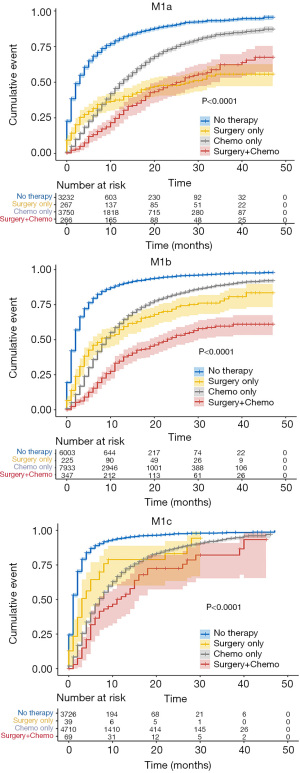
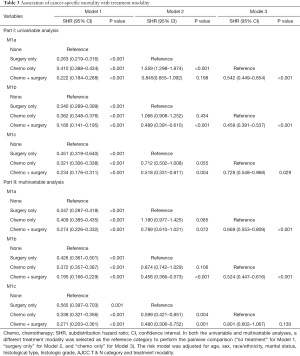
Full table
Discussion
In 2015, using a database including 94,708 patients diagnosed as lung cancer from 1999 to 2010, the International Association for the Study of Lung Cancer (IASLC) recommended a new TNM classification (23). Totally, 1,059 NSCLC patients were utilized to evaluate prognostic value of distant metastasis and develop a new M classification including M1a, M1b, and M1c (24). Patients with single extra-thoracic metastatic lesion and multiple extra-thoracic metastatic lesions were classified as M1b and M1c, respectively. Nevertheless, this classification was constructed by a univariable analysis and lacked of external validation. Therefore, we conducted a retrospective analysis using SEER database with methods of multivariable adjusted analysis and subgroup analyses. The aim of the current study was to validate the prognostic value of the proposed M classification, evaluate the prognostic effects of local consolidative therapy for stage IV NSCLC patients, and divide these patients into different subcategories to stratify the prognoses.
The current study pay attention to the association between the organs involvement of metastatic disease and the CSM rates in stage IV NSCLC patients. It was found that liver and brain involvement were independently related to high CSM rates. In addition, five subcategories were further subdivided and were found to have significantly different cumulative incidence rates of CSM across five groups. Our study indicated that selected patients would obtain benefit from local consolidative therapy and further M1 stage division may help to establish therapies.
Only a small proportion of patients with oligometastatic NSCLC have long-term disease-free intervals. Local treatment, including surgery and radiation, improved the overall survival of these patients in several retrospective studies (25-27). Furthermore, several prospective phase II clinical trials also suggested improved progression-free survival with local consolidative therapy including surgery and stereotactic body radiation therapy (SBRT) for patients with oligometastatic NSCLC (28-30). Recently, a meta-analysis including 943 patients reported that 95% of patients with oligometastatic cancer who received surgery and SBRT had local control at one year (31). The above findings are consistent with the results in the current study. In this article, we also demonstrate that chemotherapy plus surgery can improve the survival of stage IV patients, which strengthens the prognostic impact of local consolidative therapy.
There are several possible mechanisms which may explain the benefit of chemotherapy on the subject of CSM rates. First, chemotherapy may reduce the burden of malignant cells which are difficult to be eliminated by maintenance therapy and may become a source of metastatic spread. Chemotherapy would lessen the burden of malignant cells in that situation. Second, certain chemotherapies have been reported to enhance antitumor immune responses and may improve prognosis of patients (32,33). Third, the growth of distant micro-metastatic disease was promoted by residual tumor after initial systemic therapy through proangiogenic and immunosuppressive effects. In that situation, chemotherapy would slow the growth rate of distant micro-metastasis by reducing the burden of residual tumor. Remarkably, the above mechanisms are not exclusive, and the benefits of chemotherapy may result from more than one of these mechanisms.
Platinum-based chemotherapy used to be first-line therapy for advanced NSCLC lacking targetable mutations. However, immunotherapy has changed the current systemic therapy landscape. For patients with tumor programmed-cell death ligand 1 (PD-L1) expression of 50% or higher, pembrolizumab or atezolizumab monotherapy improved OS versus doublet chemotherapy (34). In another trial, pembrolizumab plus chemotherapy significantly improved the survival of patients with metastatic nonsquamous NSCLC without EGFR or ALK mutations (35) and patients with previously untreated metastatic squamous NSCLC (36). A survival benefit of atezolizumab plus chemotherapy and bevacizumab was also observed in patients with PD-L1-unselected, advanced, nonsquamous NSCLC (37).
Similar to prior studies (38-40), this study discovered bone, brain, and liver involvement were independently related to an unfavorable prognosis for stage IV NSCLC patients. According to SHR value, the absence or presence of liver involvement was identified as an more important prognostic factor for CSM rates compared with bone and brain involvement. Therefore, we supplemented the current M1 staging and grouped patients with liver involvement metastases into new categories owing to their relatively unfavorable prognoses.
It is of clinical importance to distinguish M1b from M1c disease with the reasons that (I) M1c disease tends to have higher CSM than M1b disease and (II) some patients with M1b NSCLC can benefit from local consolidative therapy. A favorable outcome was observed to be associated with local consolidative therapy in the patients with oligometastatic NSCLC (41-43). Nevertheless, the value of surgery for M1c patients was not confirmed in the current study, which may result from the high heterogeneity of organs with metastases. Given that the prior studies concerning this issue only retrospectively contained a small number of patients (25,44,45), a prospective clinical trial is necessary to be conducted.
Our study has limitations. Data on smoking status, recurrence-free survival, driver gene mutations, the type and cycle number of chemotherapy, targeted therapy, immunotherapy, and performance score are not reported in the SEER database. In addition, our study is a retrospective study, and the nature of a retrospective analysis may lead to limited data and some selection biases; completely accounting for these limitations is impossible outside of a prospective randomized clinical trial. Future prospective and multi-institutional studies are needed to validate our conclusions. Although the SEER database captures most cancer diagnoses, it is not a population-based database, and generalizability may be limited.
In future studies, precisely defining the stage of stage IV NSCLC, its subclassification as metastatic or synchronous, and its differentiation in relation to recurrence and progression will be important to obtain comparable results based on innovative biomarkers intended to facilitate unbiased treatment allocation. In particular, this process will be important in the evolving field of immunotherapy, which is a substantial pillar in the treatment of stage IV NSCLC.
Disease subdivision within the M1 category and knowledge of liver involvement may help to inform the prognosis of patients with NSCLC and guide treatment modality.
Acknowledgments
We thank Yi Fung Chau for helping us polish our article.
Funding: This work was supported by Shanghai Municipal Health Commission (2019SY072) and Shanghai Pulmonary Hospital Research Fund (FK18001 & FKGG1805 & FK1936 & FK1904). This work was also supported by National Natural Science Foundation of China (NSFC81770091). This study was also supported by Outstanding Young Medical Talent of Rising Star in Medical Garden of Shanghai Municipal Health Commission “Dong Xie”, Standardized Management of Demonstration and Application of Clinical Diagnosis and Treatment Technology (SHDC22020218) and Multi-center clinical research project for major diseases (SHDC2020CR1021B) of Shanghai Hospital Development Center.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-1383
Peer Review File: Available at https://dx.doi.org/10.21037/atm-21-1383
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-1383). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Brody H. Lung cancer. Nature 2014;513:S1. [Crossref] [PubMed]
- Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc 2019;94:1623-40. [Crossref] [PubMed]
- Paleiron N, Bylicki O, André M, et al. Targeted therapy for localized non-small-cell lung cancer: a review. Onco Targets Ther 2016;9:4099-104. [Crossref] [PubMed]
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- McMurry TL, Shah PM, Samson P, et al. Treatment of stage I non-small cell lung cancer: What's trending? J Thorac Cardiovasc Surg 2017;154:1080-7. [Crossref] [PubMed]
- Tandberg DJ, Tong BC, Ackerson BG, et al. Surgery versus stereotactic body radiation therapy for stage I non-small cell lung cancer: A comprehensive review. Cancer 2018;124:667-78. [Crossref] [PubMed]
- Ettinger Ds Fau–Wood DE, Wood De Fau–Aisner DL, Aisner Dl Fau–Akerley W, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology.
- Doroshow DB, Herbst RS. Treatment of Advanced Non-Small Cell Lung Cancer in 2018. JAMA Oncol 2018;4:569-70. [Crossref] [PubMed]
- Hendriks LE, Dingemans AM, De Ruysscher D. Proposals for the M-descriptors of the Eight TNM Classification for Non-Small Cell Lung Cancer: Are More Data Needed? J Thorac Oncol 2016;11:e42-3. [Crossref] [PubMed]
- Shin J, Keam B, Kim M, et al. Prognostic Impact of Newly Proposed M Descriptors in TNM Classification of Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:520-8. [Crossref] [PubMed]
- Reck M, Rabe KF. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:849-61. [Crossref] [PubMed]
- Tabchi S, Kassouf E, Rassy EE, et al. Management of stage III non-small cell lung cancer. Semin Oncol 2017;44:163-77. [Crossref] [PubMed]
- Amin M, Edge S, Greene F. AJCC Cancer Staging Manual, 8th ed. New York,NY: Springer; 2017.
- Brierley J, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours, 8th ed. Oxford, UK: Wiley Blackwell; 2017.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Abdel-Rahman O. Outcomes of Surgery as Part of the Management of Metastatic Non-Small-Cell Lung Cancer: A Surveillance, Epidemiology and End Results Database Analysis. Cancer Invest 2018;36:238-45. [Crossref] [PubMed]
- Wen J, Liu D, Chen D, et al. Treatment of clinical T4 stage superior sulcus non-small cell lung cancer: a propensity-matched analysis of the surveillance, epidemiology, and end results database. Biosci Rep 2019;39:BSR20181545 [Crossref] [PubMed]
- Doll KM, Rademaker A, Sosa JA. Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database. JAMA Surg 2018;153:588-9. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer ≤ 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Solomon BM, Rabe KG, Slager SL, et al. Overall and cancer-specific survival of patients with breast, colon, kidney, and lung cancers with and without chronic lymphocytic leukemia: a SEER population-based study. J Clin Oncol 2013;31:930-7. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Giroux DJ, et al. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014;9:1618-24.
- Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2015;10:1515-22.
- Griffioen GH, Toguri D, Dahele M, et al. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer 2013;82:95-102. [Crossref] [PubMed]
- Yano T, Haro A, Yoshida T, et al. Prognostic impact of local treatment against postoperative oligometastases in non-small cell lung cancer. J Surg Oncol 2010;102:852-5. [Crossref] [PubMed]
- Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014;15:346-55. [Crossref] [PubMed]
- De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol 2012;7:1547-55. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501 [Crossref] [PubMed]
- Lehrer EJ, Singh R, Wang M, et al. Safety and Survival Rates Associated With Ablative Stereotactic Radiotherapy for Patients With Oligometastatic Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2021;7:92-106. [Crossref] [PubMed]
- Gotwals P, Cameron S, Cipolletta D, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer 2017;17:286-301. [Crossref] [PubMed]
- Mathew M, Enzler T, Shu CA, et al. Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther 2018;186:130-7. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Zhang R, Li P, Li Q, et al. Radiotherapy improves the survival of patients with stage IV NSCLC: A propensity score matched analysis of the SEER database. Cancer Med 2018;7:5015-26. [Crossref] [PubMed]
- Chen VW, Ruiz BA, Hsieh MC, et al. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer 2014;120:3781-92. [Crossref] [PubMed]
- Botticelli A, Salati M, Di Pietro FR, et al. A nomogram to predict survival in non-small cell lung cancer patients treated with nivolumab. J Transl Med 2019;17:99. [Crossref] [PubMed]
- Zhao T, Gao Z, Wu W, et al. Effect of synchronous solitary bone metastasectomy and lung cancer resection on non-small cell lung cancer patients. Oncol Lett 2016;11:2266-70. [Crossref] [PubMed]
- De Leyn P, Moons J, Vansteenkiste J, et al. Survival after resection of synchronous bilateral lung cancer. Eur J Cardiothorac Surg 2008;34:1215-22. [Crossref] [PubMed]
- Gray PJ, Mak RH, Yeap BY, et al. Aggressive therapy for patients with non-small cell lung carcinoma and synchronous brain-only oligometastatic disease is associated with long-term survival. Lung Cancer 2014;85:239-44. [Crossref] [PubMed]
- Tönnies M, Pfannschmidt J, Bauer TT, et al. Metastasectomy for synchronous solitary non-small cell lung cancer metastases. Ann Thorac Surg 2014;98:249-56. [Crossref] [PubMed]
- Fleckenstein J, Petroff A, Schäfers HJ, et al. Long-term outcomes in radically treated synchronous vs. metachronous oligometastatic non-small-cell lung cancer. BMC Cancer 2016;16:348. [Crossref] [PubMed]



