
Current usage of stereotactic body radiotherapy for oligometastatic prostate cancer in Korea: patterns of care survey (KROG 19-08)
Introduction
Cancer is one of the major leading causes of death worldwide, and prostate cancer (PC) is the second most common malignancies among men worldwide. In Korea, PC is the fourth common cancer, and its incidence has been continuously increasing since 1999: approximately 10% of patients are diagnosed at distant metastatic stage (1). In patients with metastatic hormone sensitive PC, the standard treatment is androgen deprivation therapy (ADT) alone or in combination with apalutamide, abiraterone, or docetaxel with palliative intent (2,3). Unfortunately, most patients develop castration-resistant PC (CRPC) within 5 years of diagnosis, and CRPC is considered a lethal disease due to the lack of optimal treatment, although several new drugs for CRPC have shown survival benefits (4). Against this background, recent some prospective and retrospective studies reported that local treatments such as metastasis-directed therapy (MDT) and/or prostate-directed therapy (PDT) improved the survival in oligometastatic prostate cancer (OMPC), suggesting a shift in the management of metastatic PC (5-7).
The concept of oligometastases was first proposed by Hellman and Weichselbaum in 1995. For certain tumors, the anatomy and physiology may limit or concentrate theses metastases to a single or a limited number of organs, and local modalities such as radiotherapy (RT) can improve the patients’ survival and have a curative potential (8). However, the definition of oligometastases remains ambiguous. Therefore, different study groups set their own arbitrary criteria, based on the total number of metastatic lesions and/or organs. Although a new technology, stereotactic body radiotherapy (SBRT), allows the delivery of high radiation doses, no consensus has been reached on the universally sufficient radiation doses to ablate oligometastases (9). The emerging interest in OMPC led to the increasing application of SBRT with a potentially curative intent; its patterns of practice vary widely in the absence of high-level evidence.
Therefore, the Korean Stereotactic Radiosurgery Group of the Korean Society for Radiation Oncology (KOSRO) conducted a national patterns-of-care survey to better understand the patterns of curative-intent SBRT practice for OMPC in Korea. We present the following article in accordance with the SURGE reporting checklist (available at https://dx.doi.org/10.21037/atm-21-1116).
Methods
A 20-item questionnaire was sent by an e-mail to 326 radiation oncologists, who are full members of KOSRO, at 93 institutions in Korea in October 2019. The questionnaire was based on their clinical experience with OMPC and SBRT. Only 1 physician per institution was required to complete the survey sent by e-mail within 1 month. We selected one survey by order of arrival when we received multiple replies from 1 institution at the same time. Subsequently, the second survey was sent to 64 radiation oncologists in 64 institutions who had clinical experience in SBRT. The second survey consisted of questions regarding three OMPC cases. The complete survey was returned by e-mail within 1 month. The full contents of the two surveys are available in Appendix 1 and 2. In the event of non-response, the respondents were contacted by telephone and sent e-mails in order to achieve a response rate of more than 80%. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of Soonchunhyang University College of Medicine, Bucheon (IRB No. 2019-08-023-001). The need for written informed consent from the participants was waived because this study was a survey that used retrospective data of patients who were treated and we anonymized and de-identified all records and information prior to analysis so as not to infringe any patients’ rights. This study was also conducted under the authorization and cooperation of the Korea Radiation Oncology Group (KROG 19-08).
Results
Clinical experience (number of respondents =76)
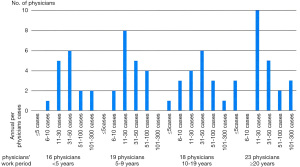
Seventy-six physicians (82%) from 93 institutions responded to the first survey. Sixteen physicians have been working as radiation oncologists for <5 years after completing residency, 19 for 5–9 years, 18 for 10–19 years, and 23 for ≥20 years. Approximately 51% of these physicians are working in tertiary referral hospitals, and 46% are working in secondary care hospitals. The multidisciplinary team approach for PC patients has been adopted in 16 institutions (21%): regularly in 7 institutions and irregularly in 9 institutions. There is a radiation oncologist as a specialist for urology in 75% of respondents. The remaining institutions have either only one radiation oncologist (13%) or a non-urologic cancer specialist (12%). The annual per-physicians cases of radical RT for PC were as follows: ≤5 cases in 4 physicians (5%), 6–10 in 6 (8%), 11–30 in 27 (35%), 31–50 in 22 (29%), 51–100 in 11 (15%), and 101–300 in 6 (8%). We presented the physicians’ working period and the annual per-physicians cases at Figure 1. The association between the physicians’ working period and SBRT experience is shown in Figure S1.
View on oligometastases (number of respondents =76)
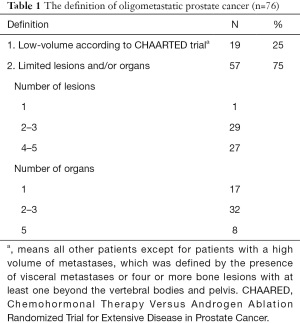
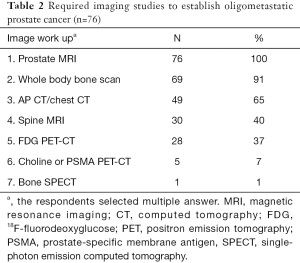
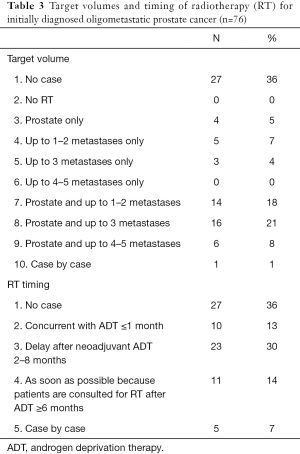
Fifty-seven respondents (75%) agreed with the definition of OMPC as 5 or less metastatic lesions and/or organs ≤5, whereas only 19 (25%) responded that oligometastases constituted a low metastatic burden based on the CHAARTED trial criteria (Table 1) (10). Physicians rely on diverse imaging studies to establish a diagnosis of OMPC (Table 2). Twenty-seven radiation oncologists (35%) had no experience treating OMPC patients with a curative intent. For the remaining 49 physicians, the annual number of OMPC patients referred for RT with curative intent are ≤5 in 49% (37 physicians), 6–10 in 11% (8), 11–20 in 4% (3), and ≥21 in 1% (1). All 49 physicians agreed on the use of RT for OMPC. The target volumes and timing of RT for patients who were initially diagnosed with OMPC varied (Table 3).
Full table
Full table
Full table
SBRT experience (number of respondents =64)
Among the 76 respondents, 12 (16%) without SBRT experience were excluded from further survey. The remaining 64 radiation oncologists with SBRT experience continued the survey. Forty-eight physicians (75%) stated that both dose and fraction number should be considered when defining SBRT, whereas 16 (25%) stated that the only fraction size should be considered. The detailed numbers are presented in Figure 2. At present, the National Health Insurance Service in Korea provides reimbursements for the cost of SBRT of up to 4 fractions only, regardless of the actually delivered fractions. Six radiation oncologists (9%) are contented with the current reimbursement schemes, whereas 58 physicians (91%) opined that the insurance should cover more than 4 fractions.
SBRT for OMPC (number of respondents =64)
In the past year, the most common fractionation schemes of PDT for OMPC were as follows: hypofractionated RT in 27 (42%), SBRT in 6 (9%), conventional fractionated RT in 5 (8%), and case-by-case basis in 5 (8%) [21 physicians (33%) had no case]. The most common fractionation schemes for MDT were as follows: SBRT in 26 (40%), hypofractionated RT in 11 (17%), case-by-case basis in 4 (6%), conventional fractionated RT in 1 (2%), and no MDT for OMPC in 1 (2%). The respondent stated that the use of SBRT for OMPC was hampered by the lack of suitable patients for SBRT (n=36, 56%), preference for other fractionation (n=21, 33%), and reimbursement issues (n=9, 14%), when allowed to select multiple answers. The pattern of PDT using SBRT is presented in Table 4. Most radiation oncologists did not use any immobilization device for prostate SBRT, and the preferred method for target localization was kilovoltage or megavoltage cone beam computed tomography. All physicians obtained the images before every treatment, and the image registration workflow during and after treatment varied (Table 4).
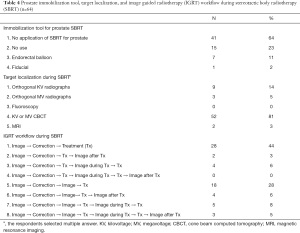
Full table
Clinical cases (number of respondents =55)
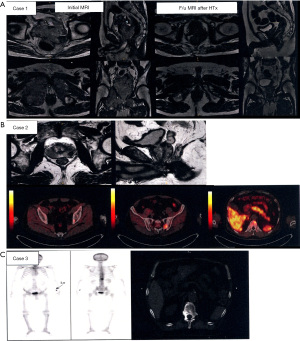
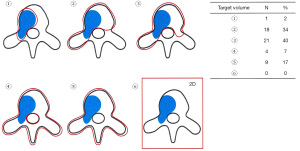
Of 64 physicians with SBRT experience, 55 (86%) responded to the second survey. Details of the three clinical cases are shown in Figure 3. They generally agreed that the three cases were categorized as OMPC to varying degrees: 49 respondents for case 1, 44 for case 2, and 55 for case 3. For case 1, 25 respondents selected to treat the whole pelvis, including regional lymph node (LN) chains and pelvic bone metastases, and 20 treated both the prostate and metastatic lesions. For case 2, 24 the respondents selected to treat the prostate and metastatic lesions, while 16 treated the whole pelvis. For case 3, all physicians treated only the metastatic lesion, but the target volume varied, as shown in Figure 4. The most preferred fractionation scheme for PDT was hypofractionated RT for PTT, whereas that for MDT varied according to the site of metastases. Hypofractionated RT is preferred for pelvic bone metastases, while SBRT is preferred for spinal metastases. Other details are summarized in Table 5.
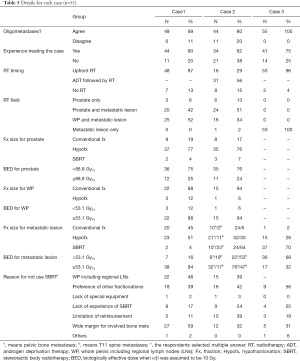
Full table
Discussion
Hellman and Weichselbaum first proposed oligometastases as an intermediate state between a localized disease and a systemically metastatic disease (8). This includes comprehensively synchronous or metachronous metastases, and controlled or uncontrolled primary tumors, regardless of the number of lesions, if all viable tumors can be treated with local modalities. Afterwards Niibe et al. (11) proposed a new definition of oligorecurrence: one to several distant metastases/recurrences (usually one) in one to several organs (usually one) with controlled primary tumor. Recently, the EORTC and ESTRO group subclassified oligometastases into synchronous oligometastases, metachronous oligorecurrence, and metachronous oligoprogression (12). Although these subclassifications would provide a clear system and reflect the fundamental biology, the exact definition of limited metastases has not yet been determined. The first phase I and randomized phase II studies, which applied SBRT as MDT for OMPC, included patients with 1–3 metastatic lesions (13,14). On the other hand, a phase III study reported that SBRT as a PDT for synchronous metastatic PC improved the overall survival of patients with a low metastatic burden according to the CHAARTED trial (15). Recent ESTRO-ASTRO consensus recommends that oligometastases can be defined as presence of 1–5 metastatic lesions (16). Meanwhile, ongoing prospective studies on OMPC can allow 5–10 metastatic lesions or unlimited metastases (17). Our survey reported that most physicians agreed that oligometastases involved a limited number of metastases, but the allowed number varied. There is a need to reach a consensus for the standardized and harmonized practice for OMPC among radiation oncologists in Korea.
A higher RT dose improves disease control in patients with localized PC, and at least 75.6 Gy conventionally fractionated RT has been established as the modern standard treatment (18). Based on the radiobiologic sensitivity to hypofractionation of PC, patient convenience, and health care costs, non-inferiority phase 3 randomized trials have confirmed the safety and efficacy of hypofractionation compared with conventional fractionation (19). Therefore, hypofractionated RT is recommended as a standard of care across all risk groups (20). SBRT is an extreme form of hypofractionation, which utilizes either a single dose or a small number of fractions; many prospective studies reported that SBRT showed similar toxicity and non-inferior disease control compared with conventional or hypofractionated RT (21-23). Nonetheless, treatment guidelines recommend SBRT for patients with low-risk PC and is considered as an alternative treatment option for those with intermediate-risk and high-risk PC (20). RT as a PDT for OMPC was assessed in two prospective studies (15,24). The HORRAD trial initiated study with 70 Gy in 35 fractions and additionally permitted 55.76 Gy in 19 fractions. The authors pointed out that the total dose was lower than the currently applied for PC, and it was considered as a limitation of the study. The STAMPEDE trial used 36 Gy in 6 fractions or 55 Gy in 20 fractions because the standard regimen of 74 Gy in 37 fractions would be too burdensome for patients with metastatic PC. In the current survey, most physicians (42%) selected hypofractionated RT as PDT for OMPC, and only 9% used SBRT. This partially affected by the insurance coverage. Since the inclusion of intensity-modulated RT (IMRT) in the health insurance system in 2015, IMRT use increased dramatically in Korea, and PC is the third most common cancer treated with IMRT (25,26). Whereas, the national insurance policy for SBRT remained unchanged and only covers at total ≤4 fractions, thus making it difficult to apply the standard number of SBRT fractions (5–6 fractions) in patients with PC. Although SBRT is an attractive modality for delivering higher radiation doses and reducing the overall treatment time, SBRT as a PDT for OMPC should be carefully performed in clinical trials due to the low quality of evidence and limited insurance resource in Korea.
SBRT as an MDT for oligometastases has been evaluated in various organ sites from different types of primary cancers. A recent multi-institutional randomized phase II study of 1–5 oligometastases from any type of primary cancer (OMPC, 16%) compared the standard therapy with SBRT and that without SBRT as MDT (27). SBRT is associated with a 13-months increase in the overall survival and the doubling of progression-free survival, but the risk of toxicity increased, including a 5% risk of grade 5 toxicity. Phase I and II studies on SBRT as MDT for OMPC showed that ADT-free survival was longer with MDT and the quality of life was maintained after SBRT (13,14). Other prospective and retrospective studies using SBRT as MDT for OMPC reported a promising local control rate of 80–100% at 2 years (6). Although SBRT as an MDT for OMPC is effective, there is no consensus on the target volume and RT dose for metastatic lesions. Prostate cancer mainly metastasizes to the bone and LNs: bone metastases divide into spine and nonspine bone metastases. The RTOG 0631 phase II/III study was the first study to specifically describe the target volume for spinal metastases according to the extent of tumor (28). International Spine Radiosurgery Consortium Consensus published contouring guidelines for SBRT for spine metastases in more detail (29). Although there are detailed recommendations from expert consensus exist, contouring must be completed on a case-by-case basis with each case tailored to the patient’s individual clinical situation and institutional infrastructure: Figure 4. reflects this potential variation in the clinical setting. For nonspine bone metastases, significant heterogeneity for contouring exists worldwide due to the absence of guidelines (30). For LN metastases, the optimal target volume between regional LN chains or affected LN only is unclear. One study reporting that two out of three OMPC patients treated with SBRT for pelvic LN relapsed in the nodes again might support the inclusion of all regional LN chains (31). Our study showed that physicians selected the regional LN chains on case-by-case basis. Ongoing multicenter, randomized, phase 2 PEACE V-STORM trial might yield clues to the potential benefit about elective nodal approach with whole pelvis RT as an alternative to focal SBRT in OMPC (32). In addition, several studies on MDT for OMPC with newer imaging modalities will help in the selection of optimal patients for SBRT (17). In terms of RT dose, most studies used 16–20 Gy in 1 fraction or 27 Gy in 3 fractions for bone metastases and lower doses for LN metastases (6,9). The universally ablative dose should be clinically validated based on patients’ outcomes by conducting further studies.
The current study has some limitations. First, respondents recollected SBRT experiences for OMPC from the past and recall bias may have occurred. Second, we used closed-ended questions and conducted descriptive analysis to get definite and vast information because little is unknown about practical patterns for SBRT for OMPC in Korea before this study. Further surveys composed of open-ended questions should be needed to reflect the accurate clinical practice. And last, the practical patterns of the respondents for OMPC may be different from those with no response. However, this survey may have representativeness because we have achieved response rate >80% from all radiation oncologists in Korea (82% in the first survey and 86% in the second survey).
In conclusion, this is the first survey to present the practical patterns of SBRT for OMPC in Korea. Most physicians agreed on the definition of OMPC as limited lesions and/or organs, but different institutions provided their own arbitrary cutoffs. The definition of the fractionation scheme of SBRT differed among institutions. Although the target volume were various among physicians, SBRT was commonly used for spinal metastases. On the other hand, physicians preferred hypofractionated RT for the treatment of the prostate and nonspine bone metastases. Based on the findings of this survey, we should conduct additional prospective studies to standardize the practice and strengthen the evidence on the role of SBRT in OMPC.
Acknowledgments
Funding: This work was supported by the Soonchunhyang University Research Fund. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Reporting Checklist: The authors have completed the SURGE reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-1116
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-1116
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-1116). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the institutional review board of Soonchunhyang University College of Medicine, Bucheon (IRB No. 2019-08-023-001). Because of the retrospective nature, the requirement of written informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hong S, Won YJ, Park YR, et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2017. Cancer Res Treat 2020;52:335-50. [Crossref] [PubMed]
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2019;381:13-24. [Crossref] [PubMed]
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. [Internet]. European Association of Urology.; Available online: https://uroweb.org/wp-content/uploads/EAU-ESUR-ESTRO-SIOG-Guidelines-on-Prostate-Cancer-large-text-V2.pdf
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol 2017;71:630-42. [Crossref] [PubMed]
- Battaglia A, De Meerleer G, Tosco L, et al. Novel Insights into the Management of Oligometastatic Prostate Cancer: A Comprehensive Review. Eur Urol Oncol 2019;2:174-88. [Crossref] [PubMed]
- Palacios-Eito A, Béjar-Luque A, Rodríguez-Liñán M, et al. Oligometastases in prostate cancer: Ablative treatment. World J Clin Oncol 2019;10:38-51. [Crossref] [PubMed]
- Miura N, Pradere B, Mori K, et al. Metastasis-directed therapy and prostate-targeted therapy in oligometastatic prostate cancer: a systematic review. Minerva Urol Nefrol 2020;72:531-42. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013;14:e28-37. [Crossref] [PubMed]
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 2015;373:737-46. [Crossref] [PubMed]
- Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol 2010;40:107-11. [Crossref] [PubMed]
- Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol 2020;21:e18-28. [Crossref] [PubMed]
- Siva S, Bressel M, Murphy DG, et al. Stereotactic Abative Body Radiotherapy (SABR) for Oligometastatic Prostate Cancer: A Prospective Clinical Trial. Eur Urol 2018;74:455-62. [Crossref] [PubMed]
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018;36:446-53. [Crossref] [PubMed]
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018;392:2353-66. [Crossref] [PubMed]
- Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol 2020;148:157-66. [Crossref] [PubMed]
- De Bruycker A, Tran PT, Achtman AH, et al. Clinical perspectives from ongoing trials in oligometastatic or oligorecurrent prostate cancer: an analysis of clinical trials registries. World J Urol 2021;39:317-26. [Crossref] [PubMed]
- Royce TJ, Mavroidis P, Wang K, et al. Tumor Control Probability Modeling and Systematic Review of the Literature of Stereotactic Body Radiation Therapy for Prostate Cancer. Int J Radiat Oncol Biol Phys 2021;110:227-36. [Crossref] [PubMed]
- Catton CN, Lukka H, Gu CS, et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J Clin Oncol 2017;35:1884-90. [Crossref] [PubMed]
- NCCN. Prostate cancer (NCC Nguidelines version 2.2020). Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. 2020
- Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol 2019;20:1531-43. [Crossref] [PubMed]
- Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019;394:385-95. [Crossref] [PubMed]
- Jackson WC, Silva J, Hartman HE, et al. Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6,000 Patients Treated On Prospective Studies. Int J Radiat Oncol Biol Phys 2019;104:778-89. [Crossref] [PubMed]
- Boevé LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol 2019;75:410-8. [Crossref] [PubMed]
- Huh SJ, Park W, Choi DH. Recent trends in intensity-modulated radiation therapy use in Korea. Radiat Oncol J 2019;37:249-53. [Crossref] [PubMed]
- Kim E, Jang WI, Kim MS, et al. Clinical utilization of radiation therapy in Korea, 2016. J Radiat Res 2020;61:249-56. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Ryu S, Yoon H, Stessin A, et al. Contemporary treatment with radiosurgery for spine metastasis and spinal cord compression in 2015. Radiat Oncol J 2015;33:1-11. [Crossref] [PubMed]
- Cox BW, Spratt DE, Lovelock M, et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2012;83:e597-605. [Crossref] [PubMed]
- Nguyen TK, Sahgal A, Dagan R, et al. Stereotactic Body Radiation Therapy for Nonspine Bone Metastases: International Practice Patterns to Guide Treatment Planning. Pract Radiat Oncol 2020;10:e452-60. [Crossref] [PubMed]
- Decaestecker K, De Meerleer G, Lambert B, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 2014;9:135. [Crossref] [PubMed]
- De Bruycker A, Spiessens A, Dirix P, et al. PEACE V - Salvage Treatment of OligoRecurrent nodal prostate cancer Metastases (STORM): a study protocol for a randomized controlled phase II trial. BMC Cancer 2020;20:406. [Crossref] [PubMed]



