
Prognostic value of hypertension in patients with nasopharyngeal carcinoma treated with intensity-modulated radiation therapy
Introduction
Nasopharyngeal carcinoma (NPC) is endemic in certain regions, especially in southern China and Southeast Asia (1). The annual incidence of NPC is 15–50 cases per 100,000 people (2). NPC is unresectable due to its proximity to the skull base, but has high radiosensitivity. Radiation therapy remains the mainstay treatment modality for locoregionally confined stages of NPC, and the tumor, nodes, and metastases (TNM) staging system is the most reliable method for guiding treatment decisions and predicting prognosis. However, the TNM staging system chiefly classifies the extent of the disease based on anatomical information, and is inadequate for assessments of prognosis (3). Thus, it would be of great clinical value to identify novel prognostic indicators to improve outcome prediction and optimize the treatment of patients with NPC.
With an occurrence rate of 37%, hypertension has been reported to be the most common comorbidity encountered in patients with tumors (4). Many studies have suggested that hypertension is associated with an increased risk of cancer, such as renal cell carcinoma (5), breast cancer (6), and urinary bladder cancer (7). In addition, renal cancer (8), pancreatic cancer (9), and esophageal cancer patients (10) with hypertension have poorer prognoses than normotensive patients.
To the best of our knowledge, only 1 study has examined the association between hypertension and the survival of NPC patients (11), and that study population covered both non-metastatic and metastatic NPC patients. As the biological behaviors and therapeutic principles are obviously different between these 2 groups, it is reasonable to discuss the prognostic indicators separately. Further, it is well known that intensity-modulated radiation therapy (IMRT) provides excellent local control and is now a mainstream radiation therapy (12). However, the prognostic value of hypertension in NPC patients treated with IMRT is unclear. Thus, in this study, we conducted a retrospective analysis of existing patient data to evaluate the prognostic effect of hypertension on the outcome of non-metastatic NPC patients treated with IMRT. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/atm-21-3130).
Methods
Patient cohort
We reviewed the medical records of all patients with NPC treated with IMRT at The Affiliated Cancer Hospital and Institute of Guangzhou Medical University between February 2010 and October 2016. All newly diagnosed patients who had been histologically confirmed to have this non-metastatic disease were included in this study. Ultimately, 1,057 patients were enrolled in this study. All the patients underwent a physical examination, fiberoptic examination, chest X-ray, abdominal ultrasonography, magnetic resonance imaging (MRI) of the neck and nasopharynx, and a whole-body bone scan using single-photon emission computed tomography (SPECT) before treatment. The medical records and imaging studies were analyzed retrospectively, and all patients were restaged according to the 8th edition of the Union for International Cancer Control/American Joint Committee on Cancer staging system for NPC. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Ethics Committee of The Affiliated Cancer Hospital & Institute of Guangzhou Medical University (No. 2020-73), and individual consent for this retrospective analysis was waived.
Hypertension assessment
All patient medical records were thoroughly reviewed. In our study, patients were diagnosed with pre-treatment hypertension if they had an average systolic blood pressure (SBP) ≥140 mmHg, and/or diastolic blood pressure (DBP) ≥90 mmHg (measured 3 times on different days) and were not being treated with antihypertensive drugs, or if they had a history of hypertension or were receiving antihypertension medication treatment. Hypertension was categorized as Grade 1 (BP ≥140/90 mmHg), Grade 2 (BP ≥160/100 mmHg), or Grade 3 (BP ≥180/110 mmHg).
Treatment
Radiotherapy
All patients were treated with definitive IMRT at The Affiliated Cancer Hospital and Institute of Guangzhou Medical University. A high-resolution planning computed tomography (CT) scan with contrast was taken from the vertex down to 2 cm below the sternoclavicular joint. The target volumes were delineated in accordance with the International Commission on Radiation Units and Measurements reports 50 and 62. The planning target volumes (PTVs) and planning organs at risk volumes (PRVs) were generated by adding a margin of 3 mm to the respective clinical target volumes (CTVs) and corresponding structures, such as the spinal cord and brainstem. The prescribed dose was 68–70 Gy to the PTV of the gross tumor volume of the primary (GTV-P), 64–66 Gy to the PTV of the nodal gross tumor volume (GTV-N), 60–66 Gy to the PTV of the clinical target volume-1 (CTV-1; i.e., high-risk regions), and 54–56 Gy to the PTV of the CTV-2 (i.e., low-risk regions) and CTV-N (i.e., neck nodal regions) in 30–33 fractions. All targets were treated simultaneously using the simultaneous integrated boost technique. The irradiation was delivered once daily, 5 days per week.
Chemotherapy
The institutional guidelines recommend no chemotherapy for patients in the early stage, and induction, concurrent and adjuvant chemotherapy or combined treatment for those in the locoregionally advanced stage. Induction or adjuvant chemotherapy consisted of platinum with 5-fluorouracil, platinum with taxane, or triplet therapy with platinum and 5-fluorouracil plus taxane every 3 weeks for 1 to 3 cycles. Concurrent chemotherapy consisted of platinum administered weekly or in weeks 1, 4, and 7 of radiotherapy. Deviation from the institutional guidelines occurred as a result of organ dysfunction, treatment intolerance, and/or patient refusal.
Follow-up period
The follow-up period was calculated from the first day of treatment to either the day of death or the day of the last examination. Each patient was assessed every 3 months during the first 2 years, and every 6 months for 3–5 years after radiotherapy. Endoscopy, CT, or MRI scans of the head and neck were performed every 3 months during the first year and annually for 2–5 years.
Statistical analysis
Statistical Package for Social Sciences version 22 (SPSS Inc., Chicago, IL, USA) was used for the analysis. The following endpoints were assessed: overall survival (OS), which was defined as the time from treatment to death resulting from any cause; progression-free survival (PFS), which was defined as the time from treatment to disease progression or death resulting from any cause; and locoregional relapse-free survival (LRRFS) and distant metastasis-free survival (DMFS), which were defined as the time from treatment to the first locoregional relapse and distant metastasis, respectively. Patients with missing data were not included in this study. Further, 156 (14.8%) patients were lost during the follow-up period. The data of the patients who were lost during the follow-up period were treated as censored data.
The baseline characteristics between the hypertensive and normotensive groups were compared and analyzed using a chi-square test. The actuarial rates were estimated using the Kaplan-Meier method, and the differences were compared using the log-rank test. Multivariate analyses with the Cox proportional hazards model were used to test the independent significance of different explanatory variables. Two-tailed P values <0.05 were considered statistically significant.
Results
Patients’ baseline characteristics
In total, 94/1,057 patients were found to be hypertensive. Of the 94 hypertensive patients, 42 (44.7%) had grade 1 hypertension, 30 (31.9%) had grade 2 hypertension, and 22 (23.4%) had grade 3 hypertension. The median follow-up period for the whole group was 54.1 months (range, 2.9–120.4 months).
The characteristics of the 1,057 NPC patients are summarized in Table 1. There were no differences between the 2 groups in terms of the distributions of sex, T stage, N stage, or clinical stage (P>0.05). However, the hypertension group had a higher percentage of patients who were older (P=0.000), and fewer hypertensive patients received chemotherapy and RT than normotensive patients (P=0.000).
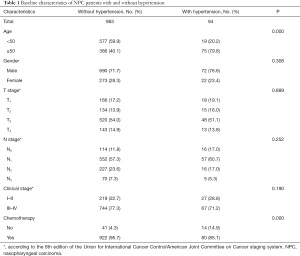
Full table
Failure pattern
The patterns of treatment failure and causes of death are shown in Table 2. Thirteen of 94 patients in the hypertension group (13.8%) and 78 of 963 patients in the normotensive group (8.1%) experienced locoregional failure, and 19 of 94 patients in the hypertension group (20.2%) and 134 of 963 patients in the normotensive group (24.3%) developed distant metastases. Additionally, 31 of 94 patients in the hypertension group (33.0%) and 134 of 963 patients in the normotensive group (13.9%) died; the majority of deaths (74.2% and 85.8%, respectively) were attributed to NPC. No significant difference was found in relation to the percentage of non-cancer-related deaths between hypertensive and normotensive patients (25.8% vs. 14.2%; P=0.115; see Table 2).
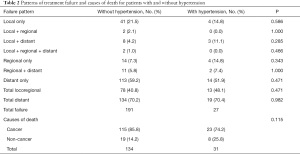
Full table
Prognostic value of hypertension in patients with NPC
For the entire patient population, the 5-year OS, PFS, LRRFS, and DMFS rates were 83.8%, 74.9%, 90.0%, and 84.5%, respectively. The treatment outcomes for normotensive and hypertensive patients were also compared. Significant differences were observed between patients with and patients without hypertension in relation to OS (66.6% vs. 85.4%, respectively; P<0.0001), PFS (60.8% vs. 76.3%, respectively; P=0.001), LRRFS (85.3% vs. 90.5%, respectively; P=0.024), and DMFS (77.4% vs. 85.1%, respectively; P=0.048). Patients without hypertension had greater treatment success rates than those with hypertension (see Figure 1 and Table 3).
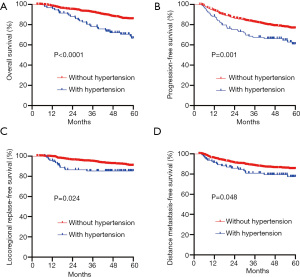
Full table
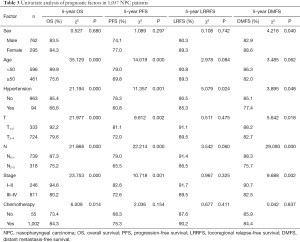
A multivariate analysis using a Cox proportional hazards model was conducted to adjust for various prognostic factors, including the following known important prognostic variables: age (<50 vs. ≥50 years), gender (male vs. female), T classification, N classification, chemotherapy (yes vs. no) and hypertension. Hypertension was identified as an independent unfavorable prognostic factor for OS [hazards ratio (HR), 2.056; 95% confidence interval (95% CI), 1.336–3.163; P=0.001], PFS (HR, 1.716; 95% CI, 1.175–2.508; P=0.005), and DMFS (HR, 1.658; 95% CI, 1.003–2.739; P=0.049). Hypertensive patients also had an increased risk of local relapse compared to normotensive patients. This difference was marginally statistically significant (HR, 1.834; 95% CI, 0.989–3.400; P=0.054). Both advanced T classification and advanced N classification were associated with an increased risk of death, disease progression and distant metastasis in the entire cohort (see Table 4).
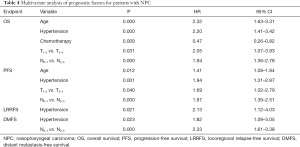
Full table
Prognostic value of the grade of hypertension
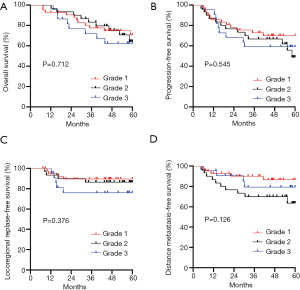
Figure 2 shows the OS, PFS, LRRFS, and DMFS curves for the hypertension groups with different grades of hypertension. In relation to the 94 NPC patients with hypertension, patients with more severe grades of hypertension had worse OS and LRRFS. Their 5-year OS and LRRFS for grade 1, 2, and 3 were 70.6%, 64.3%, and 62.4% (P=0.712), and 89.5%, 86.4%, and 76.1% (P=0.376), respectively; however, the difference was not statistically significant.
Discussion
To our knowledge, this is the largest and most detailed study undertaken to evaluate the effects of hypertension on the prognosis of non-metastatic NPC patients treated with IMRT. We found that hypertension was associated with a significant increase in the risk of death, disease progress, and distant metastasis in non-metastatic NPC patients treated with IMRT. Further, these results remained unchanged after adjusting for known prognostic factors.
In our study, the incidence of hypertension was 8.9%, which was lower than that of a previous study (17.8%) (11). A possible explanation for this difference may be the stricter patient selection criteria employed in the present study whereby only non-metastatic NPC patients who received IMRT were included in this study. The hypertensive patients in our study tended to be elderly and have only received radiotherapy. This is likely because hypertension is a kind of comorbidity, and the risk of comorbidity increases with age, and older people with complications are less able to receive chemotherapy than the rest of the population.
Various potential mechanisms have been reported to link hypertension to carcinogenic processes. Chronic hypoxia and vascular endothelial growth factor (VEGF) are considered partly responsible for the increased cancer mortality of patients with hypertension (10,13). It is well known that hypertension leads to angiosclerosis and artery stenosis, resulting in insufficient blood supply to tissues and hypoxia. Hypoxic tumor cells exhibit increased resistance to radiotherapy, which may result in treatment failure, local relapse, and metastasis (14,15), especially for NPC, which is mainly treated by radiation therapy Conversely, VEGF was found to be increased in hypertensive patients (16), and was also confirmed to promote tumor angiogenesis (17). Thus, it is possible that VEGF causes tumor cell proliferation and metastasis in cancer patients with hypertension. Further, studies have reported that there is a close relationship between hypoxia and VEGF expression in tumors and that hypoxia can induce an increase in VEGF expression (18,19). Our results indicate that NPC patients with hypertension had HRs of 2.056, 1.716, and 1.658 for death, disease progress, and distant metastasis, respectively. Our results are similar to those reported by other research groups, including those of Yang et al. (11), who conducted a multivariate analysis that indicated that hypertension is an independent risk factor of survival, and results in poorer survival outcomes in patients with NPC. Similarly, Eytan et al. (20) analyzed the effects of hypertension on the survival of head and neck cancer patients and found a 7–19% increased risk of cancer mortality in patients with hypertension compared to patients without hypertension. To date, only 2 studies (including this study) have reported the poor prognostic value of hypertension in NPC patients. More research should be conducted to evaluate the effects of hypertension on the survival outcomes of NPC patients.
Previous research has reported that the severity of hypertension can significantly affect prognosis (11). Stocks et al. (9) found that the risk of cancer mortality increased with an HR of 1.12 (95% CI, 1.08–1.15) for men and 1.06 (95% CI, 1.02–1.11) for women for every 10 mmHg increase in hypertension. Harding et al. (21) also noted a 23% increased risk for mortality in those with the highest grade of hypertension compared to those with the lowest. We examined the effects according to the grades of hypertension and found that OS and LRRFS decreased gradually with an increase in the grades of hypertension, but the difference was not statistically significant. Further investigations need to be conducted, as the non-significant difference in survival in our study seemed to be driven by the small sample size of cases with different grades of hypertension among NPC patients.
Previous findings have suggested that β-blockers have a potentially favorable prognostic role for several tumors (22,23). Due to the small sample size of the hypertension group in this study, we did not classify antihypertensive drugs or analyze the effects of different antihypertensive drugs on the prognosis of NPC. It remains unclear whether antihypertensive treatments improve cancer survival because of the blood pressure-lowering effects alone or because of additional anticancer mechanisms. Thus, this issue requires further investigation.
The results of the current study provide the first evidence of the unfavorable prognostic effect of hypertension on the prognosis of patients with non-metastatic NPC who received IMRT. Thus, researchers conducting clinical trials in patients with NPC should pay attention to the incidence of hypertension in different treatment arms. High BP can damage arteries and kidneys, causing stroke, kidney disease and other illnesses, and may result in a reduced ability to receive chemotherapy and an increase in non-cancer-related death rates. It is current practice in the tumor population to control BP within reasonable limits before antitumor therapy, which is similar to the treatment approach adopted for the non-cancer population.
It is well-known that intensity-modulated radiotherapy (IMRT) provides satisfactory long-term survival for non-metastasis NPC. Mao et al. reported that the 5-years local relapse-free survival (LRFS), nodal relapse-free survival (RRFS), DMFS, PFS, and OS rates were 94.6%, 97.0%, 82.6%, 75.1%, and 82.0%, respectively, which were similar with our research result (24). TNM staging system remains the main prognostic factors for NPC. Our results showed that both advanced TNM stage and hypertension was independent adverse prognostic factor in NPC patients treated with IMRT. Several studies have showed that Epstein-Barr virus (EBV) DNA was associated with various survival outcomes in NPC (25,26). Other biomarkers such as primary tumor volume, lactate dehydrogenase (LDH) also have demonstrated their prognostic value and potential clinical applications in NPC (27,28).
This study had some limitations. First, due to the retrospective nature of the study, a non-randomized study design was adopted. However, we attempted to reduce any potential bias by conducting univariate and multivariate analyses. Second, we only focused on patients diagnosed with hypertension who have not yet received anticancer treatment. Some patients may have become hypertensive during the follow-up period, but such patients were not included in this study, as we could not accurately determine the reliability of information gathered from outpatient or telephone follow-up conversations. Third, we did not have information about the type or dose of antihypertensive medications that participants were taking, and thus could not explore the role of specific antihypertensive medications on prognosis.
Conclusions
In the current study, hypertension was found to be an independent poor prognostic factor for non-metastatic NPC patients receiving IMRT. The question of whether the severity of hypertension affects prognosis needs to be further verified by large sample data.
Acknowledgments
Funding: This work was supported by grants from the WU JIEPING Medical Foundation (No. 320.6750.2020-13-11).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-3130
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-3130
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-3130). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The Affiliated Cancer Hospital & Institute of Guangzhou Medical University (No. 2020-73), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol 2002;13:1007-15. [Crossref] [PubMed]
- Wee JT, Ha TC, Loong SL, et al. Is nasopharyngeal cancer really a "Cantonese cancer"? Chin J Cancer 2010;29:517-26. [Crossref] [PubMed]
- Guo R, Tang LL, Mao YP, et al. Proposed modifications and incorporation of plasma Epstein-Barr virus DNA improve the TNM staging system for Epstein-Barr virus-related nasopharyngeal carcinoma. Cancer 2019;125:79-89. [Crossref] [PubMed]
- Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004;291:2441-7. [Crossref] [PubMed]
- Sanfilippo KM, McTigue KM, Fidler CJ, et al. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension 2014;63:934-41. [Crossref] [PubMed]
- Han H, Guo W, Shi W, et al. Hypertension and breast cancer risk: a systematic review and meta-analysis. Sci Rep 2017;7:44877. [Crossref] [PubMed]
- Kok VC, Zhang HW, Lin CT, et al. Positive association between hypertension and urinary bladder cancer: epidemiologic evidence involving 79,236 propensity score-matched individuals. Ups J Med Sci 2018;123:109-15. [Crossref] [PubMed]
- Grossman E, Messerli FH, Boyko V, et al. Is there an association between hypertension and cancer mortality? Am J Med 2002;112:479-86. [Crossref] [PubMed]
- Stocks T, Van Hemelrijck M, Manjer J, et al. Blood pressure and risk of cancer incidence and mortality in the metabolic syndrome and cancer project. Hypertension 2012;59:802-10. [Crossref] [PubMed]
- Liang J, Li G, Xu J, et al. Hypertension predicts a poor prognosis in patients with esophageal squamous cell carcinoma. Oncotarget 2018;9:14068-76. [Crossref] [PubMed]
- Yang P, Elhalawani H, Shi Y, et al. A large-scale retrospective study of the overall survival outcome in nasopharyngeal carcinoma with hypertension in Chinese population. Oncotarget 2017;8:75577-86. [Crossref] [PubMed]
- Du T, Xiao J, Qiu Z, et al. The effectiveness of intensity-modulated radiation therapy versus 2D-RT for the treatment of nasopharyngeal carcinoma: A systematic review and meta-analysis. PLoS One 2019;14:e0219611 [Crossref] [PubMed]
- Sharifi N, Farrar WL. Perturbations in hypoxia detection: a shared link between hereditary and sporadic tumor formation? Med Hypotheses 2006;66:732-5. [Crossref] [PubMed]
- Carlson DJ, Yenice KM, Orton CG. Tumor hypoxia is an important mechanism of radioresistance in hypofractionated radiotherapy and must be considered in the treatment planning process. Med Phys 2011;38:6347-50. [Crossref] [PubMed]
- Björk-Eriksson T, West C, Karlsson E, et al. Tumor radiosensitivity (SF2) is a prognostic factor for local control in head and neck cancers. Int J Radiat Oncol Biol Phys 2000;46:13-9. [Crossref] [PubMed]
- Belgore FM, Blann AD, Li-Saw-Hee FL, et al. Plasma levels of vascular endothelial growth factor and its soluble receptor (SFlt-1) in essential hypertension. Am J Cardiol 2001;87:805-7. [Crossref] [PubMed]
- Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019;176:1248-64. [Crossref] [PubMed]
- Morfoisse F, Kuchnio A, Frainay C, et al. Hypoxia induces VEGF-C expression in metastatic tumor cells via a HIF-1alpha-independent translation-mediated mechanism. Cell Rep 2014;6:155-67. [Crossref] [PubMed]
- Jensen RL, Ragel BT, Whang K, et al. Inhibition of hypoxia inducible factor-1alpha (HIF-1alpha) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J Neurooncol 2006;78:233-47. [Crossref] [PubMed]
- Eytan DF, Blackford AL, Eisele DW, et al. Prevalence of Comorbidities and Effect on Survival in Survivors of Human Papillomavirus-Related and Human Papillomavirus-Unrelated Head and Neck Cancer in the United States. Cancer 2019;125:249-60. [Crossref] [PubMed]
- Harding JL, Sooriyakumaran M, Anstey KJ, et al. Hypertension, Antihypertensive Treatment and Cancer Incidence and Mortality: A Pooled Collaborative Analysis of 12 Australian and New Zealand Cohorts. J Hypertens 2016;34:149-55. [Crossref] [PubMed]
- Baek MH, Kim DY, Kim SO, et al. Impact of Beta Blockers on Survival Outcomes in Ovarian Cancer: A Nationwide Population-Based Cohort Study. J Gynecol Oncol 2018;29:e82 [Crossref] [PubMed]
- Zhao Y, Wang Q, Zhao X, et al. Effect of antihypertensive drugs on breast cancer risk in female hypertensive patients: Evidence from observational studies. Clin Exp Hypertens 2018;40:22-7. [Crossref] [PubMed]
- Mao YP, Tang LL, Chen L, et al. Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin J Cancer 2016;35:103. [Crossref] [PubMed]
- Lee VH, Kwong DL, Leung TW, et al. The addition of pretreatment plasma EpsteinBarr virus DNA into the eighth edition of nasopharyngeal cancer TNM stage classification. Int J Cancer 2019;144:1713-22.
- Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet 2019;394:64-80. [Crossref] [PubMed]
- Guo R, Sun Y, Yu X-L, et al. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother Oncol 2012;104:294-9. [Crossref] [PubMed]
- Zhou GQ, Tang LL, Mao YP, et al. Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys 2012;82:e359-65. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


