Childhood cancer: an emerging public health issue in China
On page 176 of the volume 363 of Cancer Letter, Zheng and colleagues described the incidence, mortality and survival of childhood cancer in 2010 and their trends during 2000-2010 period in China mainland (1). This is a population-based study with a large number of sample size, including approximately 158 million population based on 145 (out of 219) Chinese cancer registries. Although not all registries were included, the potential implications of such an epidemic description on childhood cancer in a rapidly developing large country of China are huge. It would help the health department to make new policies and strategies on the prevention and control of childhood cancer in response to this emerging public health concern.
Childhood cancer (aka pediatric cancer) generally refers to cancer diagnosed in a child aging under 15 years old (from birth to 14 years inclusively) (2). Childhood cancer is a cause of children death just behind accidents, although it only represents between 0.5% and 4.6% of all cancers in human (3,4). Leukemia, brain tumor and lymphoma are the top three most common cancers in children, followed by less common cancers, e.g., bone, Wilms’ tumor (kidney) (1,5). Table 1 summarizes the overall incidence of childhood cancer in several different areas and/or countries with different incomes. Across the world, overall childhood cancer incidence rates range from 45 to 200 per million children. Although Uganda has a higher incidence even than developed countries, on average, the more developed countries have a higher incidence rate of childhood cancer than the less developed countries. A higher incidence of childhood cancer shown in Uganda is the most likely due to epidemic HIV infection, which has been demonstrated in association with several human cancers, such as Kaposi’s sarcoma (17,18). On the other hand, the incidence in low-income countries may have been underestimated, since many childhood cancers are not diagnosed, or are missed to be reported due to unavailable registry system (4,16). This underestimation may also happen in the rural area of China, leading to a higher incidence rate in urban than in rural area of China as reported by Zheng et al. (1). Substantial inequality of medical resources exists in China; some medical students after graduation would like to stay in big cities even with unemployment status rather than serve in the rural area, particularly the areas with poor economy.
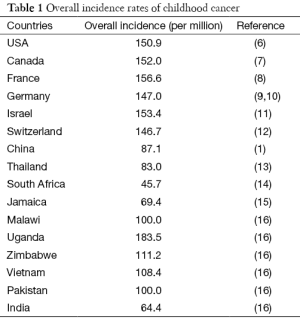
Full table
Zheng and colleagues also reported that the average annual percent change (AAPC) of incidence rate in China was 2.8% [95% confidence interval (CI), 1.1-4.6%]. It is larger than that before early 1990s in the USA (0.6%) and Europe (1.1%); and the incidence rates of childhood cancer in western countries have been shown stable since early 1990s (19-22). Improvement in diagnosis and treatment in China may be a reason to explain the rapid increase of AAPC. Another possibility may result from the environmental pollution, including air and water pollution, as well as food safety as an adverse consequence of rapid economy development in China in past decades. This postulation may also be supported by the evidence shown in Zheng et al.’s study (1) that children living in rural area had a lower incidence of childhood cancer than those living in cities.
Age and gender are two established risk factors of childhood cancer. In consistent with other previous reports, overall incidence of childhood cancer is the highest in infancy, and this rate declined to a nadir at 5-9 years old before increasing at 10-14 years old (23). This trend also is shown regardless of gender and living area (urban and rural) in China (1). Boys show slightly higher incidence in the five major childhood cancers than girls, and at all subgroups of ages (1). In addition, the positive association has been shown between parental pregnancy age and the incidence of childhood cancer (24). It is yet to be characterized whether there is any association between maternal age and childhood cancer in Zheng et al.’s study.
Ethnicity is another known risk factor of childhood cancer. Higher incidences of most types of childhood cancer are observed in Caucasian children in the US than in African, Asian and Hispanic children (23,25). In some types of childhood cancer, ethnic effect shows dramatic. For example, very few cases of Ewing sarcoma in African and Asian children, and Hispanic children show 10% higher incidence of acute leukemia than Caucasian children (23). It will be interesting to investigate whether there is any disparity across ethnicities in China, however, Zheng et al. did not further analyze the data based on race. It is unclear whether the information on race in Chinese cancer registries is available or not. Elucidation of ethnic disparity will be helpful in dissecting the role of genetic and environmental factors in childhood cancer.
Genetic factor and congenital disorders are known to associate with approximately 5-10% of childhood cancer, although the proportion of some rare cancers, such as pediatric adrenocortical carcinoma, can be higher and up to 80% of the cases carry germline TP53 mutation (26). The effect size of genetic variants in childhood cancer is usually much larger than in adult cancer, making several genome-wide association studies (GWAS) successful for childhood cancers that are relatively rare, such as Wilms’ tumor, Ewing’s sarcoma, and osteosarcoma, neuroblastoma and acute lymphoblastic leukemia (ALL) (27-31). The findings in GWAS not only show that most of phenotype-associated variants are shared across races (32), but also elucidate why some childhood cancers have a substantially higher incidence in one race than others (33). For instance, the frequencies of ALL-associated ARID5B rs10821936 are 33% in Caucasians, 24% in African-Americans, and 47% in Hispanics; and this difference may explain the lower incidence of ALL in African-Americans but higher in Hispanics (33). One pitfall of GWAS is that only common variants are included, missing rare variants with minor allele frequency (MAF) less than 0.01. However, high density GWAS available and a very large numbers of subjects easily obtained in China make it possible for researchers to analyze the effect of contiguous homozygous segments (runs of homozygosity), which are associated with rare diseases (34), on childhood cancer. Next-generation sequencing (NGS) platform and family-based study may be helpful in defining the missing genetic risk, since it is estimated that GWAS-identified common variants only explain approximately 24% of genetic variation in ALL risk (35). In addition, with the price of NGS dramatically declines, this platform will be widely applied in exploring novel rare genetic variants and their associations with human diseases including children cancer. The era of big data is on the corner, and bioinformatics and new statistical analysis methods for data mining on high dimensional data are on demand. Infrastructures and securities for big data storage are also challenging. Moreover, functional studies on identified phenotype-associated variants are another promising post-genomics topic, such as how variants affect the structure of DNA, RNA and chromatin (36,37), how variants affect RNA splicing and translation (38,39), how variants affect protein structure, modification and protein-protein interactions (40,41), and how variant-variant or gene-gene interacts or synergistically interplays (42-44).
Only some environmental factors, e.g., high dose ionizing radiation, prior chemotherapy and Epstein-Barr virus (EBV) infection, are established risk factors of childhood cancer (45-48). Most of environmental factors, however, are suggestive risk factors in childhood cancer (49-52). For instance, although the International Agency for Research on Cancer (IARC) has classified extremely low frequency magnetic field (ELF-MFs) as a possible carcinogen, a population-based case-control study conducted in Germany did not find this association between preconceptional magnetic field exposure and childhood cancer (53). Large number of environmental exposures, such as persistent organic pollutants (POPs), pesticides and other endocrine disrupters have been studied less particularly in childhood cancers (54,55). As GWAS, the term of environment-wide association study (EWAS) has been coined, which can explore the role of exposome (or exposomics, -omics of exposures) in the risk of childhood cancer. However, unlike genetic variant identification, it is challenging to accurately measure environmental exposures (e.g., parental diet, pesticide and air pollution exposure, maternal tobacco and medication usage) in determining their effects on childhood cancer risk particularly in a retrospective study design. Investigation of background levels of POPs in the varieties of food may be helpful in estimation of dietary intake. However, new markers measuring the magnitude of environmental exposures are necessary, and are a priority to elucidate the role of environmental factors in childhood cancer risk. The hypothesis of the initiation of childhood cancer in utero promotes the scheme of perinatal exposure studies (56). Figure 1 shows the potential underlying mechanisms of environmental exposure in childhood cancer. The effect of environmental exposures on children health can occur at the time windows from parental gametes to postnatal (57,58). In addition, perinatal exposures may also associate with adult diseases, which will not manifest until later in life. Longitudinal studies from birth to adult may help to identify etiology of both childhood cancer and adult diseases, in which genetic and environmental factors can be integrated to investigate the gene-environment interactions, making precise medicine rapidly progress. Furthermore, trans-generational study design will help to define the role of environmental exposures during oogenesis or parental gametes in childhood cancer (59,60).
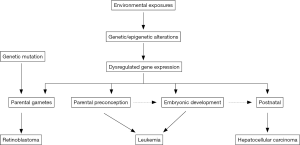
In conclusion, Zheng et al.’s study provides important information on the incidence, mortality and trends of childhood cancers in China mainland, which may help governments and policymakers to take active measurements in the prevention and control of increasing childhood cancers. This study also can emphasize the importance of comprehensive registries in the prevention and control, promoting to pay attention to disease registry regulation and the quality control of the registries across the country.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by Section Editor Hongmei Zeng, PhD (National Office for Cancer Prevention and Control, Chinese Academy of Medical Sciences, Cancer Hospital, Peking, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zheng R, Peng X, Zeng H, et al. Incidence, mortality and survival of childhood cancer in China during 2000-2010 period: A population-based study. Cancer Lett 2015;363:176-80. [PubMed]
- Bahadur G, Hindmarsh P. Age definitions, childhood and adolescent cancers in relation to reproductive issues. Hum Reprod 2000;15:227. [PubMed]
- Stewart B, Wild C. World cancer report 2014 2014. Available online: http://www.iarc.fr/en/publications/books/wcr/index.php
- Magrath I, Steliarova-Foucher E, Epelman S, et al. Paediatric cancer in low-income and middle-income countries. Lancet Oncol 2013;14:e104-16. [PubMed]
- Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev 2010;36:277-85. [PubMed]
- Li J, Thompson TD, Miller JW, et al. Cancer incidence among children and adolescents in the United States, 2001-2003. Pediatrics 2008;121:e1470-7. [PubMed]
- Mitra D, Shaw AK, Hutchings K. Trends in incidence of childhood cancer in Canada, 1992-2006. Chronic Dis Inj Can 2012;32:131-9. [PubMed]
- Lacour B, Guyot-Goubin A, Guissou S, et al. Incidence of childhood cancer in France: National Children Cancer Registries, 2000-2004. Eur J Cancer Prev 2010;19:173-81. [PubMed]
- Spix C, Eletr D, Blettner M, et al. Temporal trends in the incidence rate of childhood cancer in Germany 1987-2004. Int J Cancer 2008;122:1859-67. [PubMed]
- Kaatsch P, Spix C. Annual report 2004 (1980-2003), German childhood cancer registry. Mainz: Deutsches Kinderkrebs -register; 2004.
- Rabinowicz R, Barchana M, Liphshiz I, et al. Cancer incidence and survival among children and adolescents in Israel during the years 1998 to 2007. J Pediatr Hematol Oncol 2012;34:421-9. [PubMed]
- Michel G, von der Weid NX, Zwahlen M, et al. Incidence of childhood cancer in Switzerland: the Swiss Childhood Cancer Registry. Pediatr Blood Cancer 2008;50:46-51. [PubMed]
- Wiangnon S, Jetsrisuparb A, Komvilaisak P, et al. Childhood cancer incidence and survival 1985-2009, Khon Kaen, Thailand. Asian Pac J Cancer Prev 2014;15:7989-93. [PubMed]
- Erdmann F, Kielkowski D, Schonfeld SJ, et al. Childhood cancer incidence patterns by race, sex and age for 2000-2006: a report from the South African National Cancer Registry. Int J Cancer 2015;136:2628-39. [PubMed]
- Bishop KL, Hanchard B, Gibson TN, et al. Incidence of childhood cancer in Kingston and St Andrew, Jamaica, 1983-2002. West Indian Med J 2013;62:575-81. [PubMed]
- Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer 2008;112:461-72. [PubMed]
- Amir H, Kaaya EE, Manji KP, et al. Kaposi's sarcoma before and during a human immunodeficiency virus epidemic in Tanzanian children. Pediatr Infect Dis J 2001;20:518-21. [PubMed]
- Ziegler JL, Katongole-Mbidde E. Kaposi’s sarcoma in childhood: an analysis of 100 cases from Uganda and relationship to HIV infection. Int J Cancer 1996;65:200-3. [PubMed]
- Kaatsch P, Steliarova-Foucher E, Crocetti E, et al. Time trends of cancer incidence in European children (1978-1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006;42:1961-71. [PubMed]
- Linabery AM, Ross JA. Trends in childhood cancer incidence in the US (1992-2004). Cancer 2008;112:416-32. [PubMed]
- Baade PD, Youlden DR, Valery PC, et al. Trends in incidence of childhood cancer in Australia, 1983-2006. Br J Cancer 2010;102:620-6. [PubMed]
- Ries LAG, Smith MA, Gurney JG, et al. Cancer incidence and survival among children and adolescents: United States SEER program 1975-1995. National Cancer Institute, SEER Program, 1999 NIH Pub. no. 99-4649.
- Spector LG, Pankratz N, Marcotte EL. Genetic and nongenetic risk factors for childhood cancer. Pediatr Clin North Am 2015;62:11-25. [PubMed]
- Johnson KJ, Carozza SE, Chow EJ, et al. Parental age and risk of childhood cancer: a pooled analysis. Epidemiology 2009;20:475-83. [PubMed]
- Johnson KJ, Cullen J, Barnholtz-Sloan JS, et al. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiol Biomarkers Prev 2014;23:2716-36. [PubMed]
- Choong SS, Latiff ZA, Mohamed M, et al. Childhood adrenocortical carcinoma as a sentinel cancer for detecting families with germline TP53 mutations. Clin Genet 2012;82:564-8. [PubMed]
- Turnbull C, Perdeaux ER, Pernet D, et al. A genome-wide association study identifies susceptibility loci for Wilms tumor. Nat Genet 2012;44:681-4. [PubMed]
- Savage SA, Mirabello L, Wang Z, et al. Genome-wide association study identifies two susceptibility loci for osteosarcoma. Nat Genet 2013;45:799-803. [PubMed]
- Postel-Vinay S, Veron AS, Tirode F, et al. Common variants near TARDBP and EGR2 are associated with susceptibility to Ewing sarcoma. Nat Genet 2012;44:323-7. [PubMed]
- Diskin SJ, Capasso M, Schnepp RW, et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat Genet 2012;44:1126-30. [PubMed]
- Papaemmanuil E, Hosking FJ, Vijayakrishnan J, et al. Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat Genet 2009;41:1006-10. [PubMed]
- Marigorta UM, Navarro A. High trans-ethnic replicability of GWAS results implies common causal variants. PLoS Genet 2013;9:e1003566. [PubMed]
- Xu H, Yang W, Perez-Andreu V, et al. Novel susceptibility variants at 10p12.31-12.2 for childhood acute lymphoblastic leukemia in ethnically diverse populations. J Natl Cancer Inst 2013;105:733-42. [PubMed]
- Joshi PK, Esko T, Mattsson H, et al. Directional dominance on stature and cognition in diverse human populations. Nature 2015;523:459-62. [PubMed]
- Enciso-Mora V, Hosking FJ, Sheridan E, et al. Common genetic variation contributes significantly to the risk of childhood B-cell precursor acute lymphoblastic leukemia. Leukemia 2012;26:2212-5. [PubMed]
- Lu L, Katsaros D, Mayne ST, et al. Functional study of risk loci of stem cell-associated gene lin-28B and associations with disease survival outcomes in epithelial ovarian cancer. Carcinogenesis 2012;33:2119-25. [PubMed]
- Lu L, Risch E, Deng Q, et al. An insulin-like growth factor-II intronic variant affects local DNA conformation and ovarian cancer survival. Carcinogenesis 2013;34:2024-30. [PubMed]
- Lee LJ, Ratner E, Uduman M, et al. The KRAS-variant and miRNA expression in RTOG endometrial cancer clinical trials 9708 and 9905. PloS one 2014;9:e94167. [PubMed]
- Ratner E, Lu L, Boeke M, et al. A KRAS-variant in ovarian cancer acts as a genetic marker of cancer risk. Cancer Res 2010;70:6509-15. [PubMed]
- 1000 Genomes Project Consortium, Abecasis GR, Auton A, et al. An integrated map of genetic variation from 1,092 human genomes. Nature 2012;491:56-65.
- Zhao N, Han JG, Shyu CR, et al. Determining effects of non-synonymous SNPs on protein-protein interactions using supervised and semi-supervised learning. PLoS Comput Biol 2014;10:e1003592. [PubMed]
- Lu L, Katsaros D, Risch E, et al. Associations of LIN-28B/let-7a/IGF-II axis haplotypes with disease survival in epithelial ovarian cancer. Am J Clin Exp Obstet Gynecol 2015;2:102-15.
- Lu L, Katsaros D, Risch HA, et al. MicroRNA let-7a Modifies the Effect of Self-Renewal Gene HIWI on Patient Survival of Epithelial Ovarian Cancer. Mol Carcinog 2015. [Epub ahead of print].
- Brisson GD, Alves LR, Pombo-de-Oliveira MS. Genetic susceptibility in childhood acute leukaemias: a systematic review. Ecancermedicalscience 2015;9:539. [PubMed]
- Ron E, Modan B, Boice JD Jr, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med 1988;319:1033-9. [PubMed]
- Tucker MA, Meadows AT, Boice JD Jr, et al. Leukemia after therapy with alkylating agents for childhood cancer. J Natl Cancer Inst 1987;78:459-64. [PubMed]
- Orem J, Sandin S, Mbidde E, et al. Epstein-Barr virus viral load and serology in childhood non-Hodgkin's lymphoma and chronic inflammatory conditions in Uganda: implications for disease risk and characteristics. J Med Virol 2014;86:1796-803. [PubMed]
- Kabyemera R, Masalu N, Rambau P, et al. Relationship between non-Hodgkin's lymphoma and blood levels of Epstein-Barr virus in children in north-western Tanzania: a case control study. BMC Pediatr 2013;13:4. [PubMed]
- Wigle DT, Turner MC, Krewski D. A systematic review and meta-analysis of childhood leukemia and parental occupational pesticide exposure. Environ Health Perspect 2009;117:1505-13. [PubMed]
- Latino-Martel P, Chan DS, Druesne-Pecollo N, et al. Maternal alcohol consumption during pregnancy and risk of childhood leukemia: systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2010;19:1238-60. [PubMed]
- Klimentopoulou A, Antonopoulos CN, Papadopoulou C, et al. Maternal smoking during pregnancy and risk for childhood leukemia: a nationwide case-control study in Greece and meta-analysis. Pediatr Blood Cancer 2012;58:344-51. [PubMed]
- Rull RP, Gunier R, Von Behren J, et al. Residential proximity to agricultural pesticide applications and childhood acute lymphoblastic leukemia. Environ Res 2009;109:891-9. [PubMed]
- Hug K, Grize L, Seidler A, et al. Parental occupational exposure to extremely low frequency magnetic fields and childhood cancer: a German case-control study. Am J Epidemiol 2010;171:27-35. [PubMed]
- Bailey HD, Infante-Rivard C, Metayer C, et al. Home pesticide exposures and risk of childhood leukemia: Findings from the childhood leukemia international consortium. Int J Cancer 2015. [Epub ahead of print]. [PubMed]
- Bailey HD, Metayer C, Milne E, et al. Home paint exposures and risk of childhood acute lymphoblastic leukemia: findings from the Childhood Leukemia International Consortium. Cancer Causes Control 2015;26:1257-70. [PubMed]
- Spector LG, Hooten AJ, Ross JA. Ontogeny of gene expression: a changing environment for malignancy. Cancer Epidemiol Biomarkers Prev 2008;17:1021-3. [PubMed]
- Anderson LM, Diwan BA, Fear NT, et al. Critical windows of exposure for children's health: cancer in human epidemiological studies and neoplasms in experimental animal models. Environ Health Perspect 2000;108:573-94. [PubMed]
- Santure AW, Spencer HG. Influence of mom and dad: quantitative genetic models for maternal effects and genomic imprinting. Genetics 2006;173:2297-316. [PubMed]
- Ortega-García JA, Martin M, López-Fernández MT, et al. Transgenerational tobacco smoke exposure and childhood cancer: an observational study. J Paediatr Child Health 2010;46:291-5. [PubMed]
- Miousse IR, Currie R, Datta K, et al. Importance of investigating epigenetic alterations for industry and regulators: An appraisal of current efforts by the Health and Environmental Sciences Institute. Toxicology 2015;335:11-9. [PubMed]


