
Effects of ligustrazine on the expression of neurotransmitters in the trigeminal ganglion of a rat migraine model
Introduction
Migraine is a severely disabling neurological disorder with profound effects on productivity and health-related quality of life, which causes significant personal and societal burdens (1-3). The pathophysiology of migraine is not completely understood and there has been relatively little basic research conducted on migraine, so the understanding of and available treatment options for this condition are limited. While current preventive therapies are effective to some degree, novel and safer treatments have a positive and meaningful impact on the life of migraine patients.
Ligustrazine is the main components of Ligusticum wallichii, which has been used in the treatment of headache caused by various factors for hundreds of years in traditional Chinese medicine (TCM) (4). Although some studies have been conducted to determine the underlying mechanism of ligustrazine in treating lumbar intervertebral disc degeneration, atherosclerosis, and asthma, no study has been designed particularly regarding its mechanism in the treatment of migraine. Furthermore, little information is available on the functions of ERK, a uremic toxin aggravated the renal oxidative damage promoter, and c-fos (5), a novel urinary kidney biomarker, in migraine. In recent years, P2X3 receptors (6,7) and TRPV1 receptors (8,9) have attracted more attention in the pathogenesis of migraine attacks. Thus, in order to evaluate the protective action of ligustrazine, and the expression of P2X3 and TRPV1 receptors, ERK, and c-fos in the trigeminal nerve of a rat migraine model, we detected migraine rat trigeminal sensory neurons of P2X3 receptors, TRPV1 receptors, the ERK signaling pathway, and c-fos. We present the following article in accordance with the ARRIVE reporting checklist (available at https://dx.doi.org/10.21037/atm-21-3423).
Methods
Drug preparation
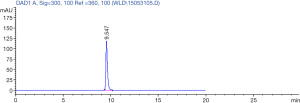
Ligustrazine hydrochloride injection was purchased from Shanghai Modern Hasen Pharmaceutical Co., Ltd. (Shanghai, China), which had >98% purity, as determined by high performance liquid chromatography (Figure 1). Nitroglycerin (NTG) was obtained from the Guangzhou Baiyunshan Pharmaceutical Co., Ltd. (Guangzhou, Guangdong, China).
Animals and treatments
Adult Wistar rats (15 males and 15 females, body weight of 200–250 g) were purchased from the Laboratory Animal Center at the Southern Medical University (Guangzhou, China). The rats were housed in a 12-h light-dark cycle and given water and food ad libitum. All experiments were conducted under the guidance of the care and use of laboratory animals issued by the Ministry of Science and Technology of the China (10). A modified version of the NTG model described by Lai (11) was used. The rats were randomly divided into three groups (n=10/group): control, migraine (model), and migraine + ligustrazine (TMP). The rats were treated for 7 consecutive days with the daily doses of ligustrazine hydrochloride injections (group TMP, 16 mL/kg/d, 10 mL sterile saline, 40 mg ligustrazine hydrochloride) or normal saline (control and model groups). At 10 min after administration of the last injection, 15 mg/kg body weight of NTG was subcutaneously administered into rat buttocks to establish an experimental migraine model (11) in the model group and TMP group.
All drug solutions were freshly prepared on the day of use. All rats were weighed before behavioral observation throughout the study. No significant differences were found in weight gain between rats treated with ligustrazine. Experiments were performed under a project license (No.: 20170301006) granted by the Laboratory Animal Ethics Committee Jinan University, in compliance with the guidance of the care and use of laboratory animals issued by the Ministry of Science and Technology of the China. A protocol was prepared before the study without registration.
Behavioral observation
At 3–5 min after the injection, the rats exhibited characteristics of head discomfort such as binaural redness, frequent forelimb scratching of head, increased attempts to climb the cage, tail biting, and reciprocating motion. The animal’s behavior, such as the climbing cage frequency, scratching head frequency, and the duration of ear redness, was continuously recorded by an observer.
Immunofluorescence
After 4 h of modeling in each group rats, 5 rats from each group were anesthetized with 10% chloral hydrate and perfusion-fixed with 4% paraformaldehyde. Their trigeminal ganglions (TGs) were isolated as previously described, and were then paraffin-embedded. Sections were dewaxed and hydrated, subjected to heat antigen retrieval, followed by endogenous peroxidase, and blocked with 5% solution of bovine serum albumin (BSA) for 15 min to block nonspecific antigen. Afterwards, the sections were incubated overnight with rabbit polyclonal anti-fos (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-P2X3 (1:500, Santa Cruz, USA) or rabbit polyclonal anti-TRPV1 (1:500, Santa Cruz, USA). The sections were then incubated for 2 h with mouse anti-rabbit Alexa Fluor 594 as secondary antibodies (1:500, Santa Cruz, USA). Between each of the links, the sections were rinsed with phosphate-buffered saline (PBS) 5 min ×3 times, plus anti-quencher, cemented with neutral gum, and observed under an inverted fluorescence microscope, the presence red fluorescent protein particles was recorded, and the control group with PBS was used as a labeled antibody.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted from cells using an RNA extraction kit (DongSheng Biotech, Guangzhou, Guangdong, China), according to the manufacturer’s instructions. Total RNA (1 µg) was then used for reverse transcription (RT) with a commercially available kit (RevertAid First Strand cDNA Synthesis Kit, Fermentas (Thermo Fisher Scientific), Waltham, MA, USA). Real-time polymerase chain reaction (qRT-PCR) was performed in triplicate with an ABI Step One Plus system (Applied Biosystems, Waltham, MA, USA) and a fluorescence-labeled SYBR Green/ROX qPCR Master Mix kit (Fermentas) using specific primers. The, P2X3 and TRPV1 were detected with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) taken as an endogenous control (primer sequences are listed in Table 1). The results were analyzed with SOS2.1 software (Applied Biosystems).Gene expression was calculated from the accurate threshold cycle (Ct), which is the PCR cycle at which an increase in fluorescence from SYBR Green probes above the baseline signal can first be detected. The Ct values for GAPDH were compared with those from P2X3 and TRPV1 in each well to calculate ΔCt. Data of the treated conditions were expressed relative to the signal obtained for the average of the untreated controls by the ΔΔCt calculation. The triplicate ΔΔCt values for each sample were averaged.

Full table
Western blot analysis
At 4 h after modeling, the remaining rats from each group were sacrificed, and their TGs were collected. Total protein was extracted from TG using a protein extraction kit (Applygen Technologies, Beijing, China). Protein concentrations in tissue lysates were determined by bicinchoninic acid (BCA) Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Then, protein samples were separated on 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to nitrocellulose membranes for 80 min at 120 V. Next, membranes were blocked with Tris-buffered saline (TBS) containing 5% (w/v) fat-free milk powder for 2 h at room temperature. After being washed 3 times with TBS, the membranes were incubated with rabbit polyclonal anti-ERK (1:1,000, Santa Cruz, USA), rabbit polyclonal anti-P2X3 (1:1,000, Santa Cruz, USA) or rabbit polyclonal anti-TRPV1 (1:1,000, Santa Cruz, USA) overnight at 4 °C. After washing 3 times with PBS, the membranes were incubated for 90 min at room temperature with diluted horseradish peroxidase (HRP)-conjugated secondary anti-bodies (1:5,000, Bioworld, Dublin, OH, USA). Blots were determined using a chemiluminescence kit (Thermo Fisher Scientific, USA) and the images were captured by the ChemiDoc XRS Imaging System (Bio-Rad) and analyzed using Image Lab 5.2.1 software.
Statistical analysis
The results were expressed as mean ± standard deviation (SD). Behavioral data were analyzed with a 2-way repeated-measures analysis of variance (ANOVA). Data from gene expression and western blot analysis were analyzed using a one-way ANOVA and Tukey’s post hoc test. A P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS 16.0 software (SPSS Inc., Armonk, NY, USA).
Results
Behavioral observation
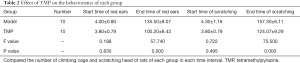
After the control group had been injected with normal saline, within 30 min scratching and climbing of the cage became a slightly conspicuous phenomenon, the rest period showed no abnormal activity, and no binaural redness was observed. At 4 min after the subcutaneous injection of NTG, both the model group and TMP group exhibited redness of the ears, frequent head scratching, cage climbing, and other phenomena, this behavior peaked at 30–90 min after modelling, and was sustained for about 2–3 hours. The duration of scratching and binaural redness of the model group were longer than those of the TMP group (P<0.001) (Table 2, Figure 2).
Full table
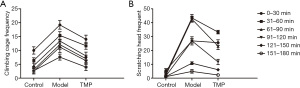
Compared with the control group, at 30 min after modelling, there were no differences in the amount of scratching between the model group and TMP group, but from 30 min onwards, the amount of cage climbing and head scratching in the TMP group were all higher than the model group over time (P<0.001), which indicated that preventive administration can significantly improve the behavioral manifestations of migraine rats (Tables 3,4, Figure 1).
Effects of ligustrazine (TMP) on the expression of P2X3 and TRPV1 in TG
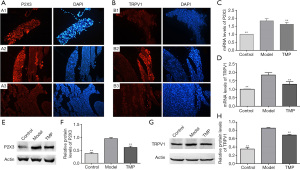
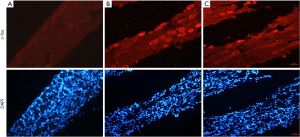
Immunofluorescence showed that the expression of P2X3 and TRPV1 in the TG neurons of all groups were mainly located in the cytoplasm (Figure 3A,3B). In the control group, there was also a small amount of positive expression in the normal state, the expression of the P2X3 and TRPV1 in the model group was substantially higher than that of the control group, while the TMP group had a lower expression than the model group. We next analyzed the messenger RNA (mRNA) expression of P2X3 and TRPV1 receptors (Figure 3C,3D); the mRNA levels in the model group were significantly higher than those in control group, while those in the TMP group were decreased in comparison to the model group. Western blot analysis further showed that P2X3 and TRPV1 level significantly were decreased in the TMP group compared to the model group (Figure 3E-3H). Thus, our data indicated that TMP can inhibit the over-expression of P2X3 and TRPV1 in TG of NTG-induced migraine rats.
Effects of ligustrazine (TMP) on the expression of c-fos in TG
Immunofluorescence results showed that in the rats TG nerve, and to varying degrees in the cytoplasm, c-fos expression showed a uniform expression status, no expression in the nucleus, and predominantly in small diameter neurons. Under normal conditions, the control group also had a small amount of expression, while expression in the model group was substantially higher than that in the control group. The expression intensity of the TMP group was reduced compared with the model group (Figure 4). The results indicated that TMP can inhibit the over-expression of c-fos in TG of NTG-induced migraine rats.
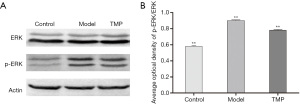
Effects of ligustrazine (TMP) on the expression of ERK protein in TG
Western blotting results of the protein expression of p-ERK/ERK in rats TG showed that it was significantly higher in the model group than in the control group, and unsurprisingly, TMP group protein expression was substantially lower than that in the model group (Figure 5). The results indicated that TMP can inhibit the over-expression of ERK protein in TG of NTG-induced migraine rats.
Discussion
The neurogenic inflammation, the cortical spreading depression (CSD) and the trigeminovascular system are the three basic mechanisms that are thought to be involved in the pathogenesis of the migraine headache. In addition, some genes are also thought to contribute to the onset of migraine, which is characterized by nausea, vomiting, sensitivity to light and sound, unilateral head pain, and attacks that are of moderate to severe pain (12). Even though pathophysiology of migraine is not fully understood, meningeal neurogenic inflammation has been largely held responsible for this state. This neuroinflammation in the meninges is referred to as sterile dural neurogenic inflammation and can be initiated by endogenous events like cortical spreading depression (13). More and more evidences show that intracellular regulatory mechanisms and intercellular signaling plays an important role in the occurrence of headache (14). The current available information indicates an intracranial network activation that culminates in the sensitization of the trigeminovascular system, release of inflammatory markers, and initiation of meningeal-like inflammatory reaction that is sensed as headache. Genetic factors might play a significant role in deciding an individual’s susceptibility to migraine (12). Accumulating studies have demonstrated that activation and sensitization of the trigeminovascular system is the key to a migraine headache (15). Some studies have shown that the binding sites of CGRP receptor antagonists and the expression of CGRP and its receptors in the trigeminal ganglion of rhesus monkeys in primates, which also indicates that there is co localization between PACAP and CGRP. Meanwhile, PACAP and CGRP showed significant changes in chronic migraine (16,17). Recently, Chinese medicine extracts have shown increasing potential as treatments for migraine due to their analgesic effect and fewer side effects (18). Ligustrazine (tetramethylpyrazine, TMP) is the main alkaloid monomer extracted from Ligusticum wallichii, which is effective in treating migraine (19). However, the mechanisms by which Ligustrazine reduce the pain in trigeminovascular system remain little understood. This current investigation explored the effects of ligustrazine on the expression of neurotransmitters in the trigeminal ganglion of a rat migraine model.
The gene c-fos plays an extremely important role in the course of migraine (20). Expression of immediate early gene c-fos positivity is the neuronal activation marker, which is rich in expression in the spinal trigeminal nucleus caudal portion. When the cells are stimulated, c-fos nucleus is transferred to the cytoplasm and translated into fos protein. Testing the fos protein can elucidate the indirect response of the distribution of the function-related cells in the brain (21).
Recent studies have found that extracellular signal-regulated kinase (ERK) regulates the transmission of neuropathic pain a new target, and migraine is a chronic neuropathic pain condition (22,23). The ERK/MAPK participate in a variety of neural plasticity activities, such as the generation and maintenance of pain; ERK/MAPK activation is necessary for the development and maintenance of neuropathic pain. Transient receptor potential vanilloid subtype 1 (TRPV1) receptors and purinergic (P2X3) receptors, are non-selective cation channels (9,24), and they high expression of both in the small diameter sensory neurons of trigeminal sensory neurons is associated with pain. In recent years, P2X3 receptors and TRPV1 receptors have attracted increasing attention in relation to the pathogenesis of migraine. When migraine happens, the expression of trigeminal sensory neurons of P2X3 receptors and TRPV1 receptors has been shown to be significantly increased (25,26).
In order to clarify the effects of ligustrazine on the expression of neurotransmitters in the trigeminal ganglion of a rat migraine model, we detected the sensory neurons of ERK signaling pathway and c-fos in the TGs of migraine model rat. We found that ligustrazine can inhibit the behavioral changes of NTG-induced migraine rat model, as well as reduce their cage climbing per unit time, episodic frequency of head scratching, and duration of ear redness. It was shown that ligustrazine can reduce the duration and severity of a migraine headache. The expression of c-fos, ERK, P2X3, and TRPV1 in NTG-induced migraine rat TGs was abundant. Therefore, Ligustrazine can inhibit the over-expression of P2X3, TRPV1, c-fos, and ERK, p-ERK in the TG of NTG-induced migraine rats.
The results showed that ligustrazine plays a role in the treatment of migraine by inhibiting the expression of pain-related neurotransmitters in the trigeminal nervous system. The mechanism may be multi-targeted, or may inhibit the expression of upstream receptors in the whole pain transmission system. For example, NLRP3 inflammasome, L-1β and substance P content may also be potential targets. The dosage of ligustrazine was counted according to the single-dose injection used clinically in adults. We did not design different groups based on alternate doses to determine the optimal dose of ligustrazine for establishing NTG models in rats, and the toxic effect of ligustrazine treatment in NTG-induced migraine rats was not observed. Therefore, further studies are warranted.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (81774239).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-3423
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-3423
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-3423). The authors report funding support from the National Natural Science Foundation of China (81774239). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No.: 20170301006) granted by the Laboratory Animal Ethics Committee Jinan University, in compliance with the guidance of the care and use of laboratory animals issued by the Ministry of Science and Technology of the China.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bible E. Pain: Comorbidity of neuropathic pain and migraine in patients with multiple sclerosis. Nat Rev Neurol 2013;9:544. [Crossref] [PubMed]
- Burnstock G. Purinergic mechanisms and pain--an update. Eur J Pharmacol 2013;716:24-40. [Crossref] [PubMed]
- Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389:816-24. [Crossref] [PubMed]
- Chang CY, Kao TK, Chen WY, et al. Tetramethylpyrazine inhibits neutrophil activation following permanent cerebral ischemia in rats. Biochem Biophys Res Commun 2015;463:421-7. [Crossref] [PubMed]
- Bohár Z, Fejes-Szabó A, Tar L, et al. Evaluation of c-Fos immunoreactivity in the rat brainstem nuclei relevant in migraine pathogenesis after electrical stimulation of the trigeminal ganglion. Neurol Sci 2013;34:1597-604. [Crossref] [PubMed]
- Burnstock G. Physiopathological roles of P2X receptors in the central nervous system. Curr Med Chem 2015;22:819-44. [Crossref] [PubMed]
- Chizhmakov I, Kulyk V, Khasabova I, et al. Molecular mechanism for opioid dichotomy: bidirectional effect of µ-opioid receptors on P2X3 receptor currents in rat sensory neurones. Purinergic Signal 2015;11:171-81. [Crossref] [PubMed]
- Christoph T, Bahrenberg G, De Vry J, et al. Investigation of TRPV1 loss-of-function phenotypes in transgenic shRNA expressing and knockout mice. Mol Cell Neurosci 2008;37:579-89. [Crossref] [PubMed]
- Flynn R, Chapman K, Iftinca M, et al. Targeting the transient receptor potential vanilloid type 1 (TRPV1) assembly domain attenuates inflammation-induced hypersensitivity. J Biol Chem 2014;289:16675-87. [Crossref] [PubMed]
- Meents JE, Neeb L, Reuter U. TRPV1 in migraine pathophysiology. Trends Mol Med 2010;16:153-9. [Crossref] [PubMed]
- Lai T, Chen L, Chen X, et al. Rhynchophylline attenuates migraine in trigeminal nucleus caudalis in nitroglycerin-induced rat model by inhibiting MAPK/NF-кB signaling. Mol Cell Biochem 2019;461:205-12. [Crossref] [PubMed]
- Khan J, Asoom LIA, Sunni AA, et al. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed Pharmacother 2021;139:111557 [Crossref] [PubMed]
- Kursun O, Yemisci M, van den Maagdenberg AMJM, et al. Migraine and neuroinflammation: the inflammasome perspective. J Headache Pain 2021;22:55. [Crossref] [PubMed]
- Messlinger K, Russo AF. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia 2019;39:1661-74. [Crossref] [PubMed]
- Chen ST, Wu JW. A new era for migraine: The role of calcitonin gene-related peptide in the trigeminovascular system. Prog Brain Res 2020;255:123-42. [Crossref] [PubMed]
- Anapindi KDB, Yang N, Romanova EV, et al. PACAP and Other Neuropeptide Targets Link Chronic Migraine and Opioid-induced Hyperalgesia in Mouse Models. Mol Cell Proteomics 2019;18:2447-58. [Crossref] [PubMed]
- Eftekhari S, Salvatore CA, Johansson S, et al. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res 2015;1600:93-109. [Crossref] [PubMed]
- Huang Y, Ni N, Hong Y, et al. Progress in Traditional Chinese Medicine for the Treatment of Migraine. Am J Chin Med 2020;48:1731-48. [Crossref] [PubMed]
- Zhen-Min XU, Min J, Xiao L, et al. Clinical practice guideline for migraine with traditional Chinese medicine(draft version for comments). Zhongguo Zhong Yao Za Zhi 2020;45:5057-67. [PubMed]
- Tang C, Unekawa M, Shibata M, et al. Characteristics of cortical spreading depression and c-Fos expression in transgenic mice having a mutation associated with familial hemiplegic migraine 2. Cephalalgia 2020;40:1177-90. [Crossref] [PubMed]
- Zhu X, Han Y, Xiong W, et al. Effects of heating coagulation of middle meningeal artery on plasma CGRP level and c-fos expression in migraine rat triggered by nitroglycerin. Neurol Sci 2011;32:589-94. [Crossref] [PubMed]
- Zhang F, Zhang Z, Kong D, et al. Tetramethylpyrazine reduces glucose and insulin-induced activation of hepatic stellate cells by inhibiting insulin receptor-mediated PI3K/AKT and ERK pathways. Mol Cell Endocrinol 2014;382:197-204. [Crossref] [PubMed]
- Zhang W, Thompson BJ, Hietakangas V, et al. MAPK/ERK signaling regulates insulin sensitivity to control glucose metabolism in Drosophila. PLoS Genet 2011;7:e1002429 [Crossref] [PubMed]
- Peng H, Zou L, Xie J, et al. lncRNA NONRATT021972 siRNA Decreases Diabetic Neuropathic Pain Mediated by the P2X3 Receptor in Dorsal Root Ganglia. Mol Neurobiol 2017;54:511-23. [Crossref] [PubMed]
- Yu L, Huang X, Huang K, et al. Ligustrazine attenuates the platelet-derived growth factor-BB-induced proliferation and migration of vascular smooth muscle cells by interrupting extracellular signal-regulated kinase and P38 mitogen-activated protein kinase pathways. Mol Med Rep 2015;12:705-11. [Crossref] [PubMed]
- Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000;288:306-13. [Crossref] [PubMed]
(English Language Editor: J. Jones)




