
The association between myocardial scar and the response of moderate ischemic mitral regurgitation to isolated coronary artery bypass grafting
Introduction
Ischemic mitral regurgitation (IMR) is a common complication of coronary artery disease (CAD) as a result of left ventricular (LV) global or regional remodeling (1,2). The estimated incidence of IMR in patients with CAD is 20–50% (2,3). IMR impairs myocardial contractility and leads to the deterioration of cardiac function, which in turn increases hospitalization for heart failure, as well as disability, mortality, and economic burden (4). The persistence of IMR significantly increases the incidence of adverse cardiovascular events in patients who have undergone coronary artery bypass grafting (CABG) (5,6). Whether mitral valve intervention should be performed in patients with moderate IMR who have received CABG remains unclear. Several randomized controlled clinical trials provide conflicting evidence (7-9), and the relevant guideline recommendations concerning the best treatment for moderate IMR lack clarity (10).
Recent studies have indicated that posterior-inferior regional remodeling, reserved ventricular function, early revascularization, large mitral leaflet size, absence of dyssynchrony between papillary muscles, and viable myocardium is associated with the improvement of moderate IMR after isolated CABG, and viable myocardium is an important factor that determined the effect of CABG on LV remodeling and the severity of IMR (11-13). Late gadolinium enhancement (LGE), determined by cardiovascular magnetic resonance (CMR), offers high spatial resolution and can detect myocardial viability and myocardial fibrosis. Myocardial replacement fibrosis (“myocardial scar”) can be used to distinguish reversible and irreversible myocardial ischemic injury (14). Theoretically, CMR-detected myocardial scar could be a potential predictor of the outcome of moderate IMR after isolated CABG, and it could be used to optimize the surgical strategy for cases of moderate IMR referred for elective CABG. In this study, we sought to evaluate whether myocardial scar assessed by CMR is a predictive factor of the outcome of moderate IMR after isolated CABG.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/atm-21-3622).
Methods
Study population
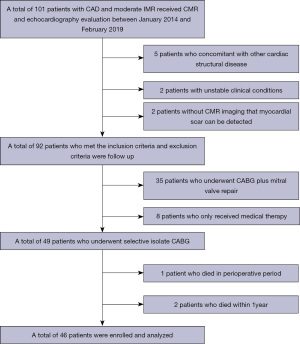
This was a case-control study. Between January 2014 and February 2019, 101 patients with CAD and moderate IMR received CMR and echocardiography evaluation in our hospital. Ninety-two patients who met the inclusion criteria and exclusion criteria were followed up and analyzed. The inclusion criteria included the following: (I) moderate IMR [effective regurgitant orifice area (EROA): 0.2–0.39 cm2, regurgitation fraction: 20–49%, and absence of organic leaflet lesions]; (II) CMR and echocardiography were performed within 10 days preoperatively; (III) myocardial scar could be accurately detected by CMR imaging. The exclusion criteria included the following: a previous history of mitral valve surgery, IMR concomitant with other cardiac structural diseases, infective endocarditis, unstable clinical conditions, cardiogenic shock during the 30 days before CABG, or ST-segment elevation myocardial infarction (MI) requiring intervention one month before surgery. Of the 92 patients, 49 underwent elective isolated CABG, 35 underwent mitral valve repair at the time of CABG, and eight patients received medical therapy. One patient (2%) subsequently died of low output syndrome and heart failure postoperatively, and two patients (4.1%) died within 12 months of surgery. Therefore, data for a total of 46 patients who had regular clinical review and echocardiography for the 12 months after surgery were retrospectively analyzed in the present study (Figure 1).
Approval from the Ethics Committee of Anzhen Hospital Affiliated to Capital Medical University was obtained before the start of the study (No. 2020100X). Because of the retrospective nature of the research, the requirement for informed consent was waived. The present study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki (as revised in 2013).
Study protocol
Preoperative clinical characteristics and echocardiography data were collected by reviewing medical information in the patients’ database. Myocardial scar was detected and analyzed with CMR imaging by an experienced radiologist. Echocardiography data were collected by reviewing outpatient electronic medical records,follow-up information such as New York Heart Association (NYHA) classification, prevalence of major adverse cardiac events, and symptom were collected by telephone interviews and outpatient reviews. According to the severity of the mitral valve regurgitation assessed by echocardiography at 12 months post-surgery, patients were classified into two groups: an improved group with no or mild IMR and an unimproved group with moderate or severe IMR. The predictors of the improvement in moderate IMR after isolated CABG were explored.
Surgical techniques
The indicators for CABG were angina associated with the left main artery, proximal left anterior descending artery (LAD), or three-vessel disease confirmed on coronary angiography. Consistently, the primary procedural choice for CABG in our institute was complete revascularization with the off-pump technique. In two cases, off-pump CABG was converted to on-pump CABG due to unstable hemodynamics. All patients achieved complete revascularization. The left internal thoracic artery was harvested and grafted to the LAD regardless of age, whereas the saphenous vein was grafted to the remaining coronary arteries. All grafts were measured by transit time flow measurement (TTFM) using Medistim VQ4122 (Medistim, Oslo, Norway). Grafts with a pulsatility index (PI) >5 and/or a mean graft flow (MGF) <10 mL/min were defined as non-functioning grafts. Re-anastomosis was mandatory for the non-functioning grafts.
Echocardiography
All patients underwent a standard transthoracic echocardiography examination with commercially available instruments within 10 days preoperatively and at 12-month follow-up. LV ejection fraction (EF), end-diastolic volume (LVEDV), and end-systolic volume (LVESV) were assessed in apical 4- and 2-chamber views with the biplane Simpson’s method. Mitral valve structure was analyzed in long- and short-axis views. The severity of mitral regurgitation was graded as follows: mild, EROA <0.2 cm2 and regurgitant fraction <30%; moderate, EROA 0.2–0.39 cm2 and regurgitant fraction 30–49%; severe, EROA >0.4 cm2 and regurgitant fraction >50%.
CMR assessment
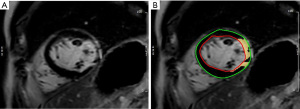
CMR images were obtained on a 3.0-T system (Verio; Siemens, Germany) using a 32-channel surface-phased array cardiac coil. Electrocardiogram-gated, balanced SSFP sequences (TrueFISP) with breath-hold were performed to obtain cine CMR images: the standard long-axis views (4-, 3-, and 2-chamber views) and contiguous short-axis views covering the entire left ventricle were used to assess LV function. The imaging parameters were as follows: repetition time, 3.50 ms; echo time, 1.51 ms; field of view, 340 mm × 289 mm. The slice thickness was 5 mm for the long axis and 8 mm for the short axis. The LGE images were acquired 10 min after intravenous administration of a gadolinium-based contrast agent (0.2 mmol/kg; Bayer, Germany) with a phase-sensitive inversion recovery (PSIR) sequence to identify the location and extent of the myocardial scar. The inversion time was set to null the signal of viable myocardium for every individual patient. The cine CMR images were analyzed using cardiovascular post-processing software (Syngo Argus; Siemens). The LGE-CMR images were analyzed using commercial cardiovascular post-processing software (Medis 3.0, Netherlands). The standard 16-segment model of the American Heart Association (AHA) was applied in the analysis of the LV myocardium (15). The LV myocardium was qualitatively analyzed, and the involved segments of MI were recorded. A summation of the volumes per slice of the areas of hyperenhancement was outlined, allowing calculation of the total infarct size (Figure 2). The myocardial scar was defined by an intensity >6 SDs higher than the user-defined viable myocardium (16). The scar percentage was automatically determined as the percentage of total myocardium (infarct mass/total LV mass). Inferior wall MI was defined as inferior wall myocardia with transmural scarring. Papillary muscle infarction (PMI) was assessed as a side-by-side reference for localizing the papillary muscle within the blood pool during the interpretation of contrast-enhanced images. PMI was considered present if any papillary hyperenhancement was present on the LGE images.
Statistical analysis
The variables are expressed as the mean ± standard deviation, median (interquartile range), or percentage, as appropriate. The Student’s t-test and the Mann-Whitney U test for independent samples were used to compare continuous variables, and the χ2 or Fisher exact test was used to compare classification variables between two groups, as appropriate. The Kaplan-Meier method was used to calculate overall survival rates between the two groups. A log-rank test was used to compare the survival curves between the two groups. Univariate and multivariate logistic regression analyses were used to assess the association between individual variables and unimproved IMR at 1-year post-CABG. Age, sex, body mass index, and variables with P<0.1 on the univariate analysis were entered into the multivariate analysis. All reported probability values were two-tailed, and a P value <0.05 was considered statistically significant. SPSS version 26.0 statistical software (IBM) and R 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria) were used for all calculations and illustrations in the present study.
Results
Baseline characteristics
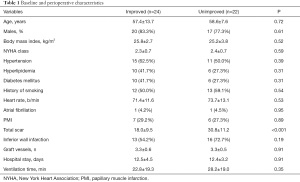
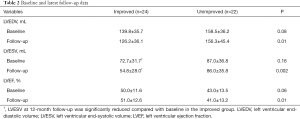
A total of 46 patients who underwent isolated CABG were included in the present study. One year after CABG, 24 patients (52.2%) had no or mild IMR (improved group), 20 patients (43.5%) had moderate IMR, and 2 patients (4.3%) had severe IMR (unimproved group). Our study cohort was middle aged (median age 58 years) and predominantly male (80.4%), with poor cardiac function (EF: 46.67%±12.91%). The mean LV total myocardial scar was 24.14%±12.13%. The prevalence of inferior wall infarction was 63.8%, and the incidence of PMI was 28.3%. There was significantly less preoperative myocardial scar in the improved group than in the unimproved group (P<0.001). There was no significant difference in other perioperative characteristics between the two groups (Table 1). Preoperative and 12-month follow-up echocardiographic data are shown in Table 2. LVEDV, LVESV, and EF were similar in the two groups at baseline. LVESV and LVEDV were significantly reduced in the improved group compared with the unimproved group at 12-month follow-up, and LVEF was also increased in the improved group. In contrast, no significant change was observed in LVESV, LVEDV, or EF in the unimproved group during the 12-month follow-up.
Full table
Full table
Predictors of unimproved IMR after isolated CABG
Factors associated with unimproved moderate IMR after CABG are shown in Table 3. Patient characteristics and clinical information, including age, sex, body mass index, LVEF, LVEDV, LVESV, myocardial scar, inferior wall MI, and PMI were included in the multivariate logistic regression analysis. Multivariate analysis revealed that only LV myocardial scar (OR: 0.89, 95% CI: 0.83–0.96, P=0.001) was a significant predictor of unimproved IMR after isolated CABG (Table 3).
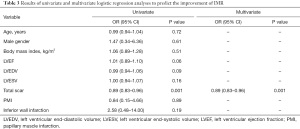
Full table
Long-term outcome
After a median follow up of 36 months (IQR, 13–73 months), two patients in the improved group and four patients in the unimproved group died. The Kaplan-Meier survival analysis revealed no difference in the predicted 3-year overall survival rates (92.3% vs. 90.3%, P=0.46) (Figure 3). However, the improved group demonstrated significant symptom relief and better ratings according to the New York Heart Association (NYHA) classification (P=0.01). Furthermore, the prevalence of major adverse cardiac events, such as MI, angina pectoris, and readmission for heart failure was significantly higher in the unimproved group (P=0.03) (Table 4).
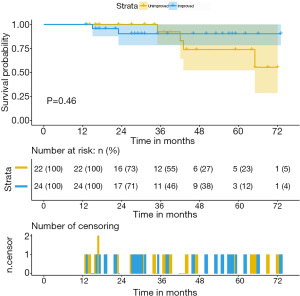

Full table
Discussion
In terms of disease-based parameters, this study showed that the extent of LV myocardial scar was an independent predictive factor of unimproved IMR after isolated CABG in patients with moderate IMR. We found that inferior wall MI, PMI, and LV function were not associated with the outcome of moderate IMR after isolated CABG. We had hoped to use receiver operating characteristic curves to detect the cutoff value of LV myocardial scar to assist in predicting unimproved IMR after isolated CABG, but this was unsuccessful due to our small sample size. Furthermore, improvement in IMR was associated with a better mid-term outcome, whereas residual IMR after isolated CABG was associated with a worse long-term outcome. Our findings suggest that scar burden might be used to optimize the surgical strategy for moderate IMR referred for elective CABG.
IMR is a common complication of MI. Approximately 30% of patients with CAD have a varying degree of mitral insufficiency after MI, and approximately 20% have moderate IMR (7,17). IMR is not a primary valve disease and is mainly caused by the remodeling of the left ventricle. IMR has been reported to be an independent predictor of mortality in heart failure patients (4). Many previous studies have revealed that IMR is a risk factor in CAD patients. Grigioni et al. reported that the presence of IMR was significantly associated with high mortality in patients with MI, which was independent of baseline characteristics and severity of ventricular dysfunction (18). Picard et al. found that both short- and long-term mortality in patients with acute cardiogenic shock had a close correlation with the degree of IMR (19). Our findings also demonstrate that improvement of IMR is associated with a better outcome, whereas unimproved IMR after CABG is associated with a worse outcome.
The indication for concomitant mitral valve repair is still controversial in patients with CAD and moderate IMR. The strategy for surgical intervention of IMR is also unclear. A 20-year retrospective study suggested that CABG alone or in combination with valve surgery could reduce mortality in patients with IMR, but combined valve surgery was not better than CABG alone (20). The Randomized Ischemic Mitral Evaluation (RIME) trial randomized patients with moderate IMR to CABG plus mitral valve repair versus CABG alone, but this trial was terminated early due to the benefit of addition mitral valve repair to CABG. The CABG plus mitral valve repair group had a greater regurgitation improvement, better functional capacity, and a decreased incidence of heart failure (8). In contrast, the Cardiothoracic Surgical Trials Network (CSTN) trial demonstrated no advantage of CABG plus mitral valve repair in reverse LV remodeling and no improvement in survival or decrease in serious adverse events (7). Several studies also found a high rate of recurrent IMR and high perioperative mortality in patients who underwent combined procedures. Therefore, these findings have failed to demonstrate a long-term survival benefit of the combined procedure (12,17). The lack of a clear consensus from these previous findings adversely affects surgical decision-making. The recommendations from the latest guidelines also provide little certainty about the best treatment for moderate IMR (10).
As noted above, there are limitations regarding best practice decisions for the treatment of moderate IMR. The primary mechanism of IMR is ischemic LV remodeling, and LV remodeling is closely related to myocardial viability. IMR is not solely due to poor leaflet coaptation or annulus dilation, whereas the myocardial viability of LV is significantly involved in the occurrence and development of IMR. Therefore, myocardial viability is a key factor in predicting LV reverse remodeling and in improving moderate IMR after isolated CABG. Penicka et al. found that reliable improvement with isolated CABG was only observed in patients who had viable myocardium and an absence of dyssynchrony between the papillary muscles (12). Kusunose et al. reported that an increased total scar burden and the presence of incomplete revascularization were powerful predictors of mortality in patients with IMR undergoing mitral valve intervention (21). The CTSN randomized study of moderate IMR suggested that improved outcome was related not only to global viability but also to the regional viability of the inferior-posterior wall (7). Kumanohoso et al. found that, compared with anterior MI, inferior MI caused a higher incidence of IMR, and more traction geometry abnormalities were found in patients with inferior MI (2). Several studies have reported a correlation between IMR and the presence of PMI (22,23). In the present study, LGE provided reliable information on myocardial viability and PM morphology. LGE permitted myocardial tissue characterization and provided measures of myocardial fibrosis. LV myocardial scar, a manifestation of myocardial viability and regional remodeling, could be accurately detected by LGE-CMR. The present study demonstrated that less preoperative LV myocardial scar was associated with improvement in moderate IMR after isolated CABG, and it was also associated with a better mid-term outcome. Inferior wall MI and PMI were not confirmed as predictors of unimproved moderate IMR after isolated CABG.
LV myocardial scar can be used to optimize the practical approach to manage patients with moderate IMR undergoing CABG. Improved IMR after isolated CABG and a better mid-term outcome appears to be reasonably predictable in patients with less preoperative LV myocardial scar. In patients with more preoperative LV myocardial scar, unimproved IMR after isolated CABG is predictable, suggesting that concomitant mitral valve repair may be necessary.
Limitations
Some limitations existed in this study. First, it was a retrospective study with a small number of patients. There are two reasons for the small sample size: (I) the number of patients with CAD and moderate IMR received LGE-CMR evaluation is small; (II) only about 50% of patients with CAD and moderate IMR underwent selective isolate CABG. So we hope more patients with moderate IMR will receive LGE-CMR evaluation to predict post-operative outcome and determine optimal surgery. For this reasons, we failed to obtain a cutoff value for LV myocardial scar to predict unimproved IMR after isolated CABG, which is considered a more robust and helpful measure when making individual surgical decisions. A larger cohort is necessary for future research. Second, most patients in our institute who underwent preoperative CMR had poor cardiac function, and 8 out of 93 patients refused surgical treatment due to the high risk. Therefore, inevitable patient selection bias existed in this study. Third, ischemia is the primary etiology of IMR, and myocardial viability plays an essential role in LV remodeling and changes to moderate IMR. A combination of LGE-CMR, positron emission tomography/computerized tomography, and echocardiography might provide more information about the outcome of moderate IMR after isolated CABG.
Conclusions
Less preoperative LV myocardial scar was present in the improved group than the unimproved group, and myocardial scar was an independent predictive factor of unimproved IMR after isolated CABG. Inferior wall MI and PMI were not confirmed as predictors of unimproved moderate IMR after isolated CABG. Unimproved IMR after isolated CABG was associated with worse mid-term outcomes.
Acknowledgments
Funding: This work was supported by Capital Medical University’s Funds for Health Improvement and Research (CHF2020-1-1053).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-3622
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-3622
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-3622). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (revised in 2013). Approval from the Ethics Committee of Anzhen Hospital Affiliated to Capital Medical University was obtained before the start of the study (No. 2020100X). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation 2005;112:745-58. [Crossref] [PubMed]
- Kumanohoso T, Otsuji Y, Yoshifuku S, et al. Mechanism of higher incidence of ischemic mitral regurgitation in patients with inferior myocardial infarction: quantitative analysis of left ventricular and mitral valve geometry in 103 patients with prior myocardial infarction. J Thorac Cardiovasc Surg 2003;125:135-43. [Crossref] [PubMed]
- Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation 2005;111:295-301. [Crossref] [PubMed]
- Grigioni F, Detaint D, Avierinos JF, et al. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. J Am Coll Cardiol 2005;45:260-7. [Crossref] [PubMed]
- Fattouch K, Sampognaro R, Speziale G, et al. Impact of moderate ischemic mitral regurgitation after isolated coronary artery bypass grafting. Ann Thorac Surg 2010;90:1187-94. [Crossref] [PubMed]
- Schroder JN, Williams ML, Hata JA, et al. Impact of mitral valve regurgitation evaluated by intraoperative transesophageal echocardiography on long-term outcomes after coronary artery bypass grafting. Circulation 2005;112:I293-8. [Crossref] [PubMed]
- Michler RE, Smith PK, Parides MK, et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med 2016;374:1932-41. [Crossref] [PubMed]
- Chan KM, Punjabi PP, Flather M, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation 2012;126:2502-10. [Crossref] [PubMed]
- Fattouch K, Guccione F, Sampognaro R, et al. POINT: Efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg 2009;138:278-85. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Sun X, Huang J, Shi M, et al. Predictors of moderate ischemic mitral regurgitation improvement after off-pump coronary artery bypass. J Thorac Cardiovasc Surg 2015;149:1606-12. [Crossref] [PubMed]
- Penicka M, Linkova H, Lang O, et al. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation 2009;120:1474-81. [Crossref] [PubMed]
- Yoshida S, Fukushima S, Miyagawa S, et al. Mitral Valve Structure in Addition to Myocardial Viability Determines the Outcome of Functional Mitral Regurgitation After Coronary Artery Bypass Grafting. Circ J 2017;81:1620-7. [Crossref] [PubMed]
- Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445-53. [Crossref] [PubMed]
- Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539-42. [Crossref] [PubMed]
- Setser RM, Bexell DG, O'Donnell TP, et al. Quantitative assessment of myocardial scar in delayed enhancement magnetic resonance imaging. J Magn Reson Imaging 2003;18:434-41. [Crossref] [PubMed]
- Mihaljevic T, Lam BK, Rajeswaran J, et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol 2007;49:2191-201. [Crossref] [PubMed]
- Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001;103:1759-64. [Crossref] [PubMed]
- Picard MH, Davidoff R, Sleeper LA, et al. Echocardiographic predictors of survival and response to early revascularization in cardiogenic shock. Circulation 2003;107:279-84. [Crossref] [PubMed]
- Castleberry AW, Williams JB, Daneshmand MA, et al. Surgical revascularization is associated with maximal survival in patients with ischemic mitral regurgitation: a 20-year experience. Circulation 2014;129:2547-56. [Crossref] [PubMed]
- Kusunose K, Obuchowski NA, Gillinov M, et al. Predictors of Mortality in Patients With Severe Ischemic Cardiomyopathy Undergoing Surgical Mitral Valve Intervention. J Am Heart Assoc 2017;6:007163 [Crossref] [PubMed]
- Bretschneider C, Heinrich HK, Seeger A, et al. Impact of Papillary Muscle Infarction on Ischemic Mitral Regurgitation Assessed by Magnetic Resonance Imaging. Rofo 2018;190:42-50. [Crossref] [PubMed]
- Bouma W, Willemsen HM, Lexis CP, et al. Chronic ischemic mitral regurgitation and papillary muscle infarction detected by late gadolinium-enhanced cardiac magnetic resonance imaging in patients with ST-segment elevation myocardial infarction. Clin Res Cardiol 2016;105:981-91. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)


