Radiation therapy to the primary tumor in locally advanced prostate cancer is not “closing the barn door after the horse has bolted”
The National Cancer Institute of Canada (NCIC) Clinical Trials Group PR.3/Medical Research Council PR07/Intergroup T94-0110 (1) was a randomized controlled trial (RCT) of radiation therapy (RT) and androgen deprivation therapy (ADT) vs. ADT alone, for men with locally advanced prostate cancer. The authors defined locally advanced as: (I) T3-4, N0/X, M0; or (II) T1-2 with prostate specific antigen (PSA) > 40 ng/mL; or (III) PSA 20-40 ng/mL and Gleason 8-10. Men were randomized to lifelong ADT vs. ADT + RT, 65-69 Gy in 1.8 Gy fractions, using 3D conformal RT, to the prostate and pelvis or prostate alone. Of the 1,205 patients treated between 1995 and 2005, 602 received ADT alone and 603 received ADT + RT. Overall survival (OS) was significantly improved in the patients allocated to ADT + RT [hazard ratio (HR) =0.70; 95% CI, 0.57-0.85; P<0.001]. Prostate cancer specific mortality (CSM) was improved in the patients allocated to ADT + RT (HR =0.46; 95% CI, 0.34-0.61; P<0.001). Although patients on ADT + RT arm reported a higher rate of gastrointestinal (GI) toxicity, only 2 of 589 patients had grade 3 or greater diarrhea at 24 months after RT.
The authors of this study should be congratulated for their work. In the early 1990s, the addition of RT to ADT in locally advanced prostate cancer was questioned, and a RCT by the Medical Research Council revealed no benefit with the addition of local therapy (2). Among various cancers (e.g., prostate, breast), treatment of a primary tumor for local control (LC), when there is suspicion of metastatic disease, was compared to “closing the barn door after the horse had bolted” (3). Critics of local therapy in the locally advanced setting emphasized the toxicity of RT: if patients were already being treated systemically with ADT, why subject them to additional toxicity of local therapy?
Strikingly, the INT T94-0110 (1,4) trial reveals a clear benefit for “closing the barn door” with local therapy, as adding RT to ADT improved OS, CSM, and freedom from biochemical failure (FFBF) compared to ADT alone [Figures 2-4, respectively (1)]. The trial underscores the importance of adding RT to ADT in high-risk, locally advanced (and possibly metastatic) prostate cancer patients. Notably, this is not the only RCT suggesting the benefit of multimodal therapy to achieve LC in locally advanced or metastatic prostate cancer (Table 1) (9). For locally advanced patients, a similar RCT from Sweden also revealed a CSM benefit for RT + ADT over ADT alone (5). Second, the French RCT (6) revealed a benefit for progression free survival, but not other outcomes. The CSM benefit was not realized in the French RCT likely due to a short median follow-up of 67 months.
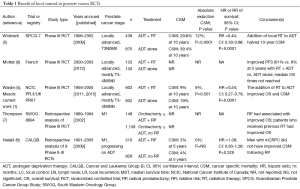
Full table
Currently, the literature is more robust for RT rather than radical prostatectomy (RP) in the locally advanced or M1 settings. Retrospective analyses of prospective trials evaluating RP in the M1 setting have revealed mixed results. For example, the Southwestern Oncology Group (SWOG) 8894 revealed a survival benefit with RP (7). The Cancer and Leukemia Group B (CALGB) did not reveal improved CSM with ADT + RP vs. RP alone (8). Similarly, a recent review article of LC in M1 patients highlighted preclinical rationale and limited high-level prospective evidence (10). It is unclear why all analyses do not reveal a benefit for LC. Significant selection bias is inherent among these unplanned subset analyses. Moreover, perhaps the benefit of LC is greatest when the extraprostatic burden of disease is the lowest (7). In support of this concept, a feasibility study exploring the potential benefit of cytoreductive RP for a men receiving ADT with oligometastatic prostate cancer demonstrated a significant improvements in PFS and CSM compared to treatment with ADT only in a carefully selected population (11).
The INT T94-0110 RCT (1) may be subject to the Will Rogers phenomenon. Most of these patients were staged M0; however, many had T3/4 disease (87%) or PSA >20 (63%) and were at high risk for subclinical metastasis. Currently, most clinicians would order computerized tomography and bone scan for these patients to rule out N1/M1 disease. We agree with the authors (1) in that their patient population represents a higher risk group than that of the Swedish group (5). The Swedish group patients had pathologic confirmation of N0 status if the PSA was >11 ng/mL (2% of the Intergroup T94-0110); 20% of patients had T1-2 disease (10% in Intergroup T94-0110); 60% of patients had a PSA of less than 20 ng/mL (37% of Intergroup T94-0110); and the maximum allowed PSA level was 70 ng/mL (unlimited in Intergroup T94-0110). Thus, in the context of the contemporary era, the INT T94-0110 RCT results can be extrapolated to imply that certain N1/M1 patients may similarly benefit from RT + ADT over ADT alone.
The INT T94-0110 RCT has certain limitations, mainly due to the era in which it was conducted. During the 1990s, dose escalation with conventional fractionation (CFRT, i.e., 1.8-2 Gy fractions, from 64 Gy to ~80 Gy) was being explored in multiple RCTs (12-18). The radiation dose that was used is now known to be inferior to the current standard of escalation to doses exceeding 74 Gy and commonly to ~80 Gy. It is possible that further dose escalation would be advantageous; as multiple RCTs and subsequent meta-analyses revealed a BF benefit of radiation dose escalation (19-21).
Additionally, the INT T94-0110 study (1) used CFRT with 3D conformal RT and elective pelvic lymph node RT. The use of intensity modulated RT is associated with fewer toxicities than 3D-CRT (12). Moreover, the benefit of pelvic lymph node RT remains unclear and is currently under investigation in Radiation Therapy Oncology Group 0924.
Next, the INT T94-0110 study does not report patient race or demographics. Studies reveal that Asian subgroups have better CSM than non-Hispanic white patients (22), that African Americans harbor a biomarker signature that portends a poor prognosis (23). With respect to toxicities and quality of life, certain subpopulations (particularly minorities) are susceptible to increased toxicity (24), and the INT T94-0110 arms may have not been balanced with respect to these subpopulations.
FFBF was one of the few endpoints to track disease progression. There is unfortunately no use of follow-up imaging to report location of failure (local vs. distant), and to track the extent of intra- vs. extra-prostatic disease. As of 2015, newer imaging modalities (including NaF positron emission tomography and multiparametric magnetic resonance imaging) would be able to quantify intraprostatic vs. extraprostatic disease recurrence and guide clinicians in recommending further focal or systemic therapy (25). The combination of advanced imaging and increasingly effective therapies for disease progression is likely to improve oncologic outcomes and confound the independent benefits of local and systemic therapies.
Finally, the use of life-long ADT is questioned in the contemporary era. Studies published since the 2000s have suggested that ADT worsens cardiovascular risk factors (obesity, type 2 diabetes mellitus, and dyslipidemia). These risk factors may lead to increased risk of cardiac mortality, which would decrease the OS benefit of both arms of INT T94-0110 (26,27).
While INT T94-0110 provides additional supporting evidence for local therapy for men with locally advanced and high-risk prostate cancers, a very intriguing aspect of this study is the predominance of high-risk features among the study population and the likelihood that current staging technologies would identify metastatic disease in large proportion of the study population. To further clarify the role of local therapy in patients presenting with metastatic disease, a prospective, multi-institutional, randomized, phase II trial of best systemic therapy vs. best systemic therapy plus definitive treatment (RT or RP) of the primary tumor in M1 prostate cancer is currently recruiting patients (NCT01751438). Best systemic therapy includes ADT, secondary hormonal therapies, chemotherapy, tyrosine kinase inhibitors, and/or immunotherapy. RP may use a variety of surgical approaches (e.g., robotic assisted, radical retropubic prostatectomy, radical cystoprostatectomy, total pelvic exenteration); RT may use IMRT, 3D-CRT, or proton therapy. A maximum of 120 patients will be randomized with 1:1 ratio between the arms. Progression will be defined by PSA response or change in lesion size on imaging. If RP and RT have similar efficacy, PFS should be improved with either treatment, and there should not be a CSM difference between the two arms; OS may depend on other factors, including patient comorbidities.
In conclusion, the INT T94-0110 study (1) demonstrates a substantive benefit of LC with RT in locally advanced prostate cancer patients, and provides the suggestion of potential benefit in those with micrometastatic or limited metastatic disease. Although this is not the only study to suggest a benefit of LC, it is one of the few studies that is prospective and randomized. Compared to contemporary techniques, INT T94-0110 did not employ an “adequate” dose of RT (i.e. ≥74 Gy), it used older RT techniques, it did not differentiate local vs. distant recurrence, and it did not contain patient-specific data (e.g., genetics, demographics or comorbidities). These differences may all be key factors in identifying the subset of patients where LC is most important.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by Bo Fan, MD, PhD (Department of Urology, the First Affiliated Hospital of Dalian Medical University, Dalian, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mason MD, Parulekar WR, Sydes MR, et al. Final Report of the Intergroup Randomized Study of Combined Androgen-Deprivation Therapy Plus Radiotherapy Versus Androgen-Deprivation Therapy Alone in Locally Advanced Prostate Cancer. J Clin Oncol 2015;33:2143-50. [PubMed]
- Fellows GJ, Clark PB, Beynon LL, et al. Treatment of advanced localised prostatic cancer by orchiectomy, radiotherapy, or combined treatment. A Medical Research Council Study. Urological Cancer Working Party--Subgroup on Prostatic Cancer. Br J Urol 1992;70:304-9. [PubMed]
- Morrow M, Goldstein L. Surgery of the primary tumor in metastatic breast cancer: closing the barn door after the horse has bolted? J Clin Oncol 2006;24:2694-6. [PubMed]
- Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet 2011;378:2104-11. [PubMed]
- Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009;373:301-8. [PubMed]
- Mottet N, Peneau M, Mazeron JJ, et al. Addition of radiotherapy to long-term androgen deprivation in locally advanced prostate cancer: an open randomised phase 3 trial. Eur Urol 2012;62:213-9. [PubMed]
- Thompson IM, Tangen C, Basler J, et al. Impact of previous local treatment for prostate cancer on subsequent metastatic disease. J Urol 2002;168:1008-12. [PubMed]
- Halabi S, Vogelzang NJ, Ou SS, et al. The impact of prior radical prostatectomy in men with metastatic castration recurrent prostate cancer: a pooled analysis of 9 Cancer and Leukemia Group B Trials. J Urol 2007;177:531-4. [PubMed]
- Zaorsky NG, Trabulsi EJ, Lin J, et al. Multimodality therapy for patients with high-risk prostate cancer: current status and future directions. Semin Oncol 2013;40:308-21. [PubMed]
- Bayne CE, Williams SB, Cooperberg MR, et al. Treatment of the Primary Tumor in Metastatic Prostate Cancer: Current Concepts and Future Perspectives. Eur Urol 2015. [Epub ahead of print].
- Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol 2015;193:832-8. [PubMed]
- Zaorsky NG, Harrison AS, Trabulsi EJ, et al. Evolution of advanced technologies in prostate cancer radiotherapy. Nat Rev Urol 2013;10:565-79. [PubMed]
- Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:67-74. [PubMed]
- Al-Mamgani A, van Putten WL, Heemsbergen WD, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;72:980-8. [PubMed]
- Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol 2010;28:1106-11. [PubMed]
- Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol 2007;8:475-87. [PubMed]
- Beckendorf V, Guerif S, Le Prisé E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys 2011;80:1056-63. [PubMed]
- Michalski JM, Moughan J, Purdy JA, et al. Initial Results of a Phase 3 Randomized Study of High Dose 3DCRT/IMRT versus Standard Dose 3D-CRT/IMRT in Patients Treated for Localized Prostate Cancer (RTOG 0126). Int J Radiat Oncol Biol Phys 2014;90:1263.
- Hoskin PJ, Rojas AM, Bownes PJ, et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol 2012;103:217-22. [PubMed]
- Vargas CE, Martinez AA, Boike TP, et al. High-dose irradiation for prostate cancer via a high-dose-rate brachytherapy boost: results of a phase I to II study. Int J Radiat Oncol Biol Phys 2006;66:416-23. [PubMed]
- Zaorsky NG, Palmer JD, Hurwitz MD, et al. What is the ideal radiotherapy dose to treat prostate cancer? A meta-analysis of biologically equivalent dose escalation. Radiother Oncol 2015;115:295-300. [PubMed]
- Trinh QD, Nguyen PL, Leow JJ, et al. Cancer-specific mortality of Asian Americans diagnosed with cancer: a nationwide population-based assessment. J Natl Cancer Inst 2015;107:djv054. [PubMed]
- Yamoah K, Johnson MH, Choeurng V, et al. Novel Biomarker Signature That May Predict Aggressive Disease in African American Men With Prostate Cancer. J Clin Oncol 2015;33:2789-96. [PubMed]
- Kleinmann N, Zaorsky NG, Showalter TN, et al. The effect of ethnicity and sexual preference on prostate-cancer-related quality of life. Nat Rev Urol 2012;9:258-65. [PubMed]
- Zaorsky NG, Yamoah K, Thakur ML, et al. A paradigm shift from anatomic to functional and molecular imaging in the detection of recurrent prostate cancer. Future Oncol 2014;10:457-74. [PubMed]
- Keating NL, O'Malley A, Freedland SJ, et al. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 2012;104:1518-23. [PubMed]
- Nguyen PL, Chen MH, Beckman JA, et al. Influence of androgen deprivation therapy on all-cause mortality in men with high-risk prostate cancer and a history of congestive heart failure or myocardial infarction. Int J Radiat Oncol Biol Phys 2012;82:1411-6. [PubMed]


