Refractory pulmonary artery aneurysm in Behçet’s disease
Introduction
Behçet’s disease (BD) is a multisystemic vasculitis affecting all sizes of arteries and veins (1-3). Pulmonary artery aneurysm (PAA) is the most lethal complication of the disease and suggests a poor prognosis. The mean survival after the onset of hemoptysis was reported to be about 10 months (1). Therapeutic options for PAAs include medical, endovascular, and surgical therapies (1,2). The former endovascular therapies were only used for peripheral types of PAAs (4). No endovascular occlusion for pulmonary artery trunk has been reported, and no therapeutic algorithm exists for the rare disease. We describe an endovascular occlusion for a refractory PAA in BD, and provide a proposal of a therapeutic algorithm.
Case report
A 27-year-old man was referred for recurrent hemoptysis over the previous 4 years. He had been known to have BD for 5 years. He had treated with colchicine, prednisone, and thalidomide for 13 months. C-reactive protein level was 9.6 mg/L, and erythrocyte sedimentation rate was 19 mm/h. Chest radiograph revealed rounded right parahilar opacity (Figure 1A). Chest computed tomography (CT) showed a 50 mm × 45 mm right main PAA (Figure 1B). Selective pulmonary angiography confirmed the PAA in right main pulmonary artery. Bronchoscopy showed bleeding from the right main bronchus.
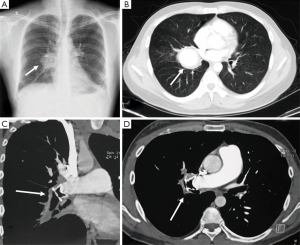
He was treated with intravenous cylophosphamide and steroids, as well as transcatheter occlusion of the right main pulmonary artery with a modified double-disk occluder. Chest CT confirmed the double-disk occluder was located at the beginning of PAA (Figure 1C,D). Hemoptysis ceased for 4 months, and then he experienced three episodes of massive hemoptysis in one week. Emergency right pneumonectomy had to be performed. We underwent intrapericardial division of right main pulmonary artery and removed the occluder. Surgical pathology revealed an aneurysm 50 mm in diameter associated with a 12 mm tuberculosis lesions in right lower lobe. The fistulous communication was between the enlarged right main pulmonary artery and right main bronchus. The patient’s postoperative course was complicated by a right main bronchopleural fistula and empyema. The right chest drain was performed, and he was kept on an oral immunosuppressive regimen. There was no hemoptysis recurrence during a 2-year follow-up.
Discussion
Therapeutic options for PAAs include medical, endovascular, and surgical therapies (1-2). When used in early stage, immunosuppressive drugs in combination with steroids may cause regression or complete disappearance of PAA. Despite medical therapy, PAA may develop to massive hemoptysis due to progressive vasculitis.
The former endovascular therapies were only used for peripheral types of PAAs to control hemoptysis. No endovascular occlusion for pulmonary artery trunk has been reported. To our knowledge, this is the first report of using amplatzer occluders to control massive hemoptysis for central types of PAA in BD. In the present case, although the endovascular therapy was failed after four months, the endovascular occlusion provided a feasible alternative therapy. The pneumonectomy supplied a salvage procedure after failures of medical and endovascular therapies.
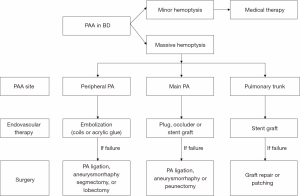
Severity of the hemoptysis, the sites and number of PAAs are essential factors in choosing therapy. We provide a therapeutic algorithm for massive hemoptysis according to bleeding sites of PAAs (Figure 2). The high mortality associated with surgery makes endovascular therapy a feasible alternative to emergency surgery. If PAAs are bilateral types, endovascular therapies or surgeries should only be applied to the bleeding side. For peripheral types of PAAs, embolizations of affected pulmonary arteries with coils or acrylic glue should be tried. For central types, plugs, amplatzer occluders or stent grafts (5) could be used in selected patients. Surgical interventions, including pulmonary artery ligation, anatomic lung resection (2) and aneurysmorrhaphy (3), should only be used for salvage therapy. Pneumonectomy should be avoided if possible, as PAAs may develop on the contralateral side.
Conclusions
PAA is a life-threatening complication of Behcet’s disease. Amplatzer occlusion could be used in the central types of PAA to control massive hemoptysis before emergency surgery. Surgical interventions should only be used for salvage therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hamuryudan V, Yurdakul S, Moral F, et al. Pulmonary arterial aneurysms in Behçet's syndrome: a report of 24 cases. Br J Rheumatol 1994;33:48-51. [PubMed]
- Tüzün H, Hamuryudan V, Yildirim S, et al. Surgical therapy of pulmonary arterial aneurysms in Behçet's syndrome. Ann Thorac Surg 1996;61:733-5. [PubMed]
- Aroussi AA, Redai M, El Ouardi F, et al. Bilateral pulmonary artery aneurysm in Behçet syndrome: report of two operative cases. J Thorac Cardiovasc Surg 2005;129:1170-1. [PubMed]
- Pelage JP, El Hajjam M, Lagrange C, et al. Pulmonary artery interventions: an overview. Radiographics 2005;25:1653-67. [PubMed]
- Park A, Cwikiel W. Endovascular treatment of a pulmonary artery pseudoaneurysm with a stent graft: report of two cases. Acta Radiol 2007;48:45-7. [PubMed]


