
The clinical value of serum hepatic parenchyma cell volume-normalized hepatitis B surface antigen levels in hepatitis B e antigen -positive and -negative chronic hepatitis B patients
Introduction
Serum hepatitis B surface antigen (HBsAg) plays an important role in the diagnosis of hepatitis B virus (HBV) infection, evaluation of disease treatment, and the assessment of hepatocellular carcinoma (HCC) risk. HBsAg clearance is the desired endpoint of antiviral therapy for patients with chronic hepatitis B (CHB) (1-3).
Persistent HBV infection is the result of interactions between virus replication and host immunity. Covalently closed circular DNA (cccDNA) is the source of hepatitis B virus (HBV) replication. HBV cannot be completely eliminated from infected hepatocytes due to the existence of intrahepatic cccDNA. Viral replication and HBsAg secretion change dynamically and there are positive correlations between intrahepatic ccc DNA and serum levels of HBV DNA and HBsAg. Serum HBsAg levels indirectly reflect viral replication and hepatocyte damage in CHB patients (4-6).
The biological functions of HBsAg and its role in the pathogenesis of CHB are not fully understood (7). Furthermore, hepatitis B e antigen (HBeAg)-positive or -negative expression can vary markedly in patients with different degrees of liver inflammation and hepatic fibrosis. In CHB patients, persistent inflammation of hepatocytes results in progressive fibrosis, leading to fewer hepatic parenchymal cells, that is, a lower hepatic parenchymal cell volume (HPCV) available for HBV replication (8). Compared with HBeAg-positive CHB, HBeAg-negative CHB is associated with lower serum levels of HBV DNA and HBsAg, but more severe hepatic inflammation and necrosis (9). However, it is not clear if the degree of fibrosis and HPCV influences circulating HBsAg levels. The paper argued HPCV-normalized serum HBsAg in HBeAg-positive and HBeAg-negative stages, which represent different periods in the natural history of chronic hepatitis B, as well as different hepatic fibrosis stages, which affects the hepatic parenchymal cell mainly.
A number of studies have suggested that markers of CHB infection, such as HBsAg levels, may more accurately indicate the stage of disease and viral replication when they are normalized to HPCV compared to using the absolute measured serum values (10,11). This is because the number of hepatic parenchymal cells available for viral replication varies with the amount of liver fibrosis. Thus, the purpose of this study was to compare absolute serum HBsAg levels and HPCV-normalized HBsAg levels in HBeAg-positive and HBeAg-negative CHB patients. We present the following article in accordance with the MDAR reporting checklist (available at https://dx.doi.org/10.21037/atm-21-3846).
Methods
Patients
Patients with CHB treated at the Department of Infectious Disease in our hospital between 2008 and 2012 were retrospectively enrolled in this study. Patients were included in if they: (I) presented with CHB infection and were treated at our institution; (II) had not received any prior antiviral treatments (to eliminate any influence of treatment on serum HBsAg levels and liver pathological diagnosis); (III) had appropriate testing to exclude hepatitis A, C, D, and E infection, and HIV infection; and (IV) had undergone a liver biopsy and pathological examination of the hepatic tissue. Liver biopsy is standard protocol at our institution for the diagnosis and evaluation of CHB infection.
Ethics statement
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethical committee of the Third Affiliated Hospital of Sun Yat-sen University (the ethical number: [2018]02-429-01). All patients provided written informed consent for all treatments performed, including the liver biopsy, and for the use of their data for scientific investigations. All patient data were fully anonymized. None of the authors of this study were involved in the treatment or management of any of the patients included in the analysis.
Serum HBsAg and other antigen levels
Serum antigen levels, including HBsAg levels, were measured with the Elecsys system (Roche Diagnostics GmbH, Mannheim, Germany) and the corresponding kits according to the manufacturer’s instructions. The reference range for HBsAg, hepatitis B surface antibody (anti-HBs), HBeAg, hepatitis B e antibody (anti-HBe), and hepatitis B core antibody (anti-HBc) were as follows: HBsAg, <1.0 cut-off index (COI); anti-HBs, 0–10 IU/L; HBeAg, <1.0 COI; anti-HBe, >1.0 COI; anti-HBc, >1.0 COI (i.e., COI values <1 were defined as negative, and COI values >1 were defined as positive).
Liver histopathological diagnosis and measurement of hepatic fibrosis
Liver biopsies were performed with an automatic biopsy gun using a 16 G needle, guided by an Esaote AU4 color Doppler system (Esaote, USA). Biopsy specimens were approximately 20 mm in length. Tissue specimens were fixed with Bouin’s solution and embedded in paraffin. Specimens were stained with hematoxylin-eosin (HE) and Masson’s trichrome (Figure 1). Hepatic tissues were examined by pathologists experienced in liver diseases.

Disease activity grade was staged using the modified histology activity index (12) and hepatic fibrosis was assessed using the Ishak fibrosis score (12).
The hepatic fibrosis proportion was measured with an automatic imaging analysis system (Kontron IBAS 2.5, Germany) using a Zeiss Axiotron microscope (Carl Zeiss AG, Germany) with a JVC-KY-F30B3-CCD lens (Japan) at 400× magnification. The percentage of fibrosis was calculated from randomly selected fields of view in the 4 corners and center of a section, and then the average of the 5 values was used for further analysis.
The results of our prior study indicated that the percentage of hepatic fibrosis in stage 1, 2, 3, and 4 of the disease is 8.31%±2.90%, 11.43%±2.76%, 14.97%±5.88%, and 20.73%±4.44%, respectively (13).
Calculation of HPCV using the proportion of fibrosis at different stages of hepatic fibrosis
The liver consists of a majority of hepatic parenchyma cells, and a minority of sinusoidal endothelial cells, Kupffer cells, and mesenchymal cells. Therefore, the HPCV is approximately equivalent to the number of hepatocytes. Liver tissues were observed under a light microscope, and the field of view was circular in shape. The circular field of view consists of different proportions of hepatic parenchyma area and hepatic fibrosis area, which when summed equal 100% of the field of view (100% rotundity). Based on our prior study (13), the proportion of hepatic parenchyma cell area in a field of view is equal to 100% of the area of each field of vision minus the area of hepatic fibrosis in the field of view. Thus, the percentage of hepatic parenchymal cell area in hepatic fibrosis stage 1 is 91.69% (100% − 8.31%), 88.57% in stage 2 (100% − 11.43%), 85.03% in stage 3 (100% − 14.97%), and 79.27% in stage 4 (100% − 20.73%). These values were used for calculations in the current study.
The rotundity area formula is A=πR2. The radii (R) of internal smaller rotundity hepatic parenchyma cell area is equal to the square root of the ratio of internal smaller rotundity hepatic parenchyma cell area (A) to pi (π), namely . The radii of the rotundity area of hepatic parenchyma cell area in hepatic fibrosis stage 1 is. In stage 2, it is, in stage 3, it is, and in stage 4, it is.
The volume of a sphere is expressed as volume (V) = 4/3πR3. Thus, the 3-dimensional (3D) sphere HPCV volumes for hepatic fibrosis stage 1–4 are as follows: Vstage 1=4/3πR13=4/3×3.14×0.54043=66.07%; Vstage 2= 4/3πR23=4/3×3.14×0.53113=62.72%, Vstage 3=4/3πR33=4/3×3.14×0.52043=59.00%; and Vstage 4=4/3πR43=4/3×3.14×0.50233=53.06%. The rationale and process of calculations are shown in Figure 1.
Calculation of HPCV-normalized serum HBsAg levels
To calculate HPCV-normalized HBsAg levels, serum HBsAg levels were divided by the HPCV percentage. This was performed as for hepatic fibrosis stages 1, 2, 3, and 4.
Statistical analysis
Statistical analyses were performed using SPSS version 20.0 software. Comparisons of absolute serum HBsAg levels and HPCV-normalized HBsAg between HBeAg-positive and HBeAg-negative CHB patients were performed using Student’s independent t-test. The correlation between serum HBsAg levels (absolute and HPCV-normalized) and liver inflammation grades or hepatic fibrosis stage was examined using Pearson’s correlation coefficient data, respectively. All analyses were 2-tailed, and a value of P<0.05 was considered to be statistically significant.
Results
The correlation between patient characteristics and liver inflammation grade and hepatic fibrosis stage
A total of 254 CHB patients were retrospectively enrolled in this study. There were 127 HBeAg-positive patients, including 104 males and 23 females, with a median age of 33.9±10.1 years. There were 127 HBeAg-negative patients, consisting of 106 males and 21 females, with a median age of 39.6±9.7 years. Liver inflammation grade was positively correlated with hepatic fibrosis stage in both HBeAg-positive (R=0.841; P<0.001) and HBeAg-negative (R=0.843; P<0.001) patients (Table 1). In the majority of patients, more severe liver inflammation was associated with more severe hepatic fibrosis.
Table 1
| Liver inflammation grades | Hepatic fibrosis stages | Total | |||
|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||
| HBeAg (+) | |||||
| G1 | 15 | 1 | 0 | 0 | 16 |
| G2 | 12 | 35 | 5 | 0 | 50 |
| G3 | 0 | 5 | 22 | 9 | 36 |
| G4 | 0 | 2 | 3 | 20 | 25 |
| Total | 27 | 41 | 30 | 29 | 127 |
| HBeAg (−) | |||||
| G1 | 29 | 3 | 0 | 1 | 33 |
| G2 | 9 | 26 | 5 | 1 | 41 |
| G3 | 0 | 1 | 17 | 9 | 27 |
| G4 | 0 | 1 | 6 | 19 | 26 |
| Total | 38 | 31 | 28 | 30 | 127 |
Correlation analysis of liver inflammation grades and hepatic fibrosis stages: HBeAg-positive, R=0.841, P<0.001; HBeAg-negative, R=0.843, P<0.001. HBeAg, hepatitis B e antigen.
Analysis of the absolute and HPCV-normalized HBsAg levels in HBeAg-positive and HBeAg-negative CHB patients
Absolute serum HBsAg levels in HBeAg-positive CHB patients were significantly higher than that observed in HBeAg-negative patients (P=0.004). However, HPCV-normalized serum HBsAg levels were not significantly different between the HBeAg-positive and the HBeAg-negative groups (P=0.071; Table 2). To confirm these results, multivariate linear regression models adjusted for patient gender, age, liver inflammation grade, and hepatic fibrosis stage were performed. The estimated regression coefficients of HBeAg status (negative as reference) with absolute serum HBsAg level (log10 COI) and HPCV-normalized serum HBsAg level (log10 COI) were −0.15 (95% CI: −0.24 to −0.05, P=0.003) and −0.25 (95% CI: −0.41 to −0.09, P=0.002), respectively.
Table 2
| HBeAg-positive CHB | HBeAg-negative CHB | t | P | |
|---|---|---|---|---|
| Serum HBsAg (log10 COI) | 3.70±0.37 | 3.56±0.38 | 2.871 | 0.004 |
| Serum HBsAg apportioned by the same HPCV (log10COI) | 6.15±0.83 | 6.00±0.88 | 1.815 | 0.071 |
HPCV, hepatic parenchyma cell volume; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; CHB, chronic hepatitis B; COI, cut-off index.
Pearson correlation analyses of serum HBsAg with liver inflammation grade and hepatic fibrosis stage in HBeAg-positive and HBeAg-negative CHB patients
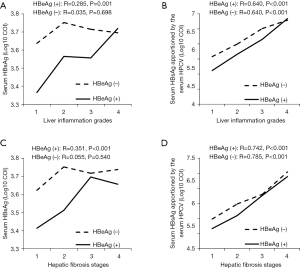
In HBeAg-positive CHB patients, absolute serum HBsAg levels correlated positively with both liver inflammation grade (R=0.285, P=0.001) and hepatic fibrosis stage (R=0.351, P<0.001). While HPCV-normalized HBsAg levels also showed a positive correlation with liver inflammation grade (R=0.640, P<0.001) and hepatic fibrosis stage (R=0.742, P<0.001) (Figure 2). In HBeAg-negative CHB patients, there was no correlation between absolute serum HBsAg levels and liver inflammation grade (R=0.035, P=0.698) nor hepatic fibrosis stage (R=0.055, P=0.540). However, there was a significant positive correlation between HPCV-normalized HBsAg levels and liver inflammation grade (R=0.640, P<0.001) and hepatic fibrosis stage (R=0.785, P<0.001) (Figure 2).
Discussion
Serum HBV DNA, HBsAg, and HBeAg levels are the most convenient and reliable indicators of viral replication in CHB patients (14). The European Association for the Study of the Liver (EASL) 2017 Clinical Practice Guidelines classified CHB infection into the following 5 stages: (I) HBeAg-positive chronic infection; (II) HBeAg-positive chronic hepatitis; (III) HBeAg-negative chronic infection; (IV) HBeAg-negative chronic hepatitis; and (V) HBsAg-negative phase (15). The seroconversion from HBeAg to HBeAb is often accompanied by HBV DNA suppression and remission of pathological damage (15). While there have been many studies examining changes of serum HBsAg and the degree of liver inflammation and hepatic fibrosis in CHB patients, clear associations have not been found (16-19). However, it should be noted that the impact of the degree of fibrosis on HPCV or the effective hepatic cell number was considered in the aforementioned studies. In the HBeAg-positive and HBeAg-negatives stage of CHB infection, liver injury is caused by inflammation and the associated fibrosis formation, and persistent inflammation results in progressive fibrosis. As fibrosis increases, the effective hepatic cell quantity (HPCV) decreases. Undoubtedly, when there are fewer hepatic parenchyma cells for host HBV replication, HBsAg production will be reduced, lowering serum HBsAg levels (20,21).
Our results showed that the absolute serum HBsAg levels were higher in HBeAg-positive CHB patients than in HBeAg-negative CHB patients. However, HPCV-normalized HBsAg levels were not different between HBeAg-positive and HBeAg-negative patients. This result suggested that HBeAg seroconversion does not truly reflect suppression of HBV replication or HBsAg synthesis, and HBeAg status need not be considered in HBV antiviral therapy. This finding is consistent with the current guidelines of HBV treatment (15) in which the HBeAg-positive and HBeAg-negative CHB patients are no longer treated differently. HBeAg may exhibit spontaneous serological conversion in some CHB patients. However, this does not necessarily mean that viral replication in the body is immunologically controlled. HBeAg seroconversion can be related to mutations of the pro-C or C promoter regions, and in this case, active viral replication will still occur (22,23). During the HBeAg-negative phase of CHB HBV replication, HBsAg expression in a single hepatocyte is not less than that observed in the HBeAg-positive phase. The mechanisms and extent of immune-mediated pathological damage are similar in HBeAg-positive and HBeAg-negative CHB.
Furthermore, our results demonstrated that in both HBeAg-positive and HBeAg-negative CHB patients, HPCV-normalized serum HBsAg levels (i.e., hepatic cell quantity) were significantly positively correlated with liver inflammation grade and hepatic fibrosis stage. However, in HBeAg-negative CHB, absolute HBsAg levels were not correlated with liver inflammation grade and hepatic fibrosis stage. This finding suggested that the “natural decline” of detectable serum HBsAg in the natural course of CHB infection with seroconversion from HBeAg-positive to HBeAg-negative does not represent a reduction or stationary phase of viral replication, nor lessening of liver tissue injury. Instead, the results suggested that HBV replication in a single hepatocyte is more active, and thus, can aggravate immune-mediated pathological injury and potentially trigger the development of tumors (24). As such, the impact of liver inflammation and hepatic fibrosis on HPCV should be considered when treating HBeAg-negative CHB patients with low or declining serum HBsAg levels. To this end, non-invasive methods for the assessment of liver fibrosis, or liver biopsies, should be recommended for HBeAg-negative CHB patients with low serum HBsAg levels and normal liver function. Effective antiviral therapy should be promptly administered based on the assessment of liver fibrosis and inflammation (25).
There were some limitations to this study. First, the HBsAg levels were not tested and reported using the worldwide recognized units IU/mL, and this is a major limitation to the present study. In addition, although HPCV-normalized HBsAg levels, rather than absolute HBsAg levels, were associated with inflammation and fibrosis in both HBeAg-positive and HBeAg-negative patients, the underlying mechanisms remain to be investigated in future studies.
Conclusions
In summary, HPCV-normalized HBsAg levels, rather than absolute HBsAg levels, were positively correlated with liver inflammation grade and hepatic fibrosis stage in both HBeAg-positive and HBeAg-negative CHB patients. HPCV-normalized HBsAg levels may provide more accurate information regarding disease status compared to absolute HBsAg levels in CHB patients.
Acknowledgments
Funding: This study was supported by the National Science and Technology Major Project (2018ZX10302204-002) and the National Natural Science Foundation of China (81672701).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-3846
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-3846
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-3846). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by by the local ethical committee of the Third Affiliated Hospital of Sun Yat-sen University (the ethical number: [2018]02-429-01). All patients provided written informed consent for all treatments performed, including the liver biopsy, and for the use of their data for scientific investigations. All patient data were fully anonymized.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Martinot-Peignoux M, Lapalus M, Asselah T, et al. The role of HBsAg quantification for monitoring natural history and treatment outcome. Liver Int 2013;33:125-32. [Crossref] [PubMed]
- Martinot-Peignoux M, Lapalus M, Asselah T, et al. HBsAg quantification: useful for monitoring natural history and treatment outcome. Liver Int 2014;34:97-107. [Crossref] [PubMed]
- Fourati S, Pawlotsky JM. Recent advances in understanding and diagnosing hepatitis B virus infection. F1000Res 2016;5:F1000 Faculty Rev-2243.
- Guner R, Karahocagil M, Buyukberber M, et al. Correlation between intrahepatic hepatitis B virus cccDNA levels and other activity markers in patients with HBeAg-negative chronic hepatitis B infection. Eur J Gastroenterol Hepatol 2011;23:1185-91. [Crossref] [PubMed]
- Locarnini S, Bowden S. Hepatitis B surface antigen quantification: not what it seems on the surface. Hepatology 2012;56:411-4. [Crossref] [PubMed]
- Zeng LY, Lian JS, Chen JY, et al. Hepatitis B surface antigen levels during natural history of chronic hepatitis B: a Chinese perspective study. World J Gastroenterol 2014;20:9178-84. [PubMed]
- Liang TJ. Hepatitis B: the virus and disease. Hepatology 2009;49:S13-21. [Crossref] [PubMed]
- Lin CL, Kao JH. New perspectives of biomarkers for the management of chronic hepatitis B. Clin Mol Hepatol 2016;22:423-31. [Crossref] [PubMed]
- Alexopoulou A, Karayiannis P. HBeAg negative variants and their role in the natural history of chronic hepatitis B virus infection. World J Gastroenterol 2014;20:7644-52. [Crossref] [PubMed]
- Ke WM, Xie SB, Li XJ, et al. There were no differences in serum HBV DNA level between HBeAg-positive and HBeAg-negative chronic hepatitis B with same liver histological necroinflammation grade but differences among grades 1, 2, 3 and 4 apportioned by the same hepatic parenchyma cell volume. J Viral Hepat 2011;18:637-45. [Crossref] [PubMed]
- Wu ZB, Cao H, Liu T, et al. Dynamic expression profile of HBsAg according to hepatic parenchyma cells' volume at different liver fibrosis stages in the immune clearance phase. Zhonghua Gan Zang Bing Za Zhi 2012;20:742-5. [PubMed]
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696-9. [Crossref] [PubMed]
- Xie SB, Yao JL, Zheng SS, et al. The levels of serum fibrosis marks and morphometric quantitative measurement of hepatic fibrosis. Hepatobiliary Pancreat Dis Int 2002;1:202-6. [PubMed]
- Diktas H, Karacaer Z, Özturk II, et al. Comparison of relationship between histopathological, serological and biochemical parameters in patients with chronic hepatitis B infection. Postgrad Med J 2016;92:693-6. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-98. [Crossref]
- Xun YH, Zang GQ, Guo JC, et al. Serum hepatitis B surface antigen quantification as a useful assessment for significant fibrosis in hepatitis B e antigen-positive hepatitis B virus carriers. J Gastroenterol Hepatol 2013;28:1746-55. [Crossref] [PubMed]
- Xie Q, Hu X, Zhang Y, et al. Decreasing hepatitis B viral load is associated with a risk of significant liver fibrosis in hepatitis B e antigen positive chronic hepatitis B. J Med Virol 2014;86:1828-37. [Crossref] [PubMed]
- Martinot-Peignoux M, Carvalho-Filho R, Lapalus M, et al. Hepatitis B surface antigen serum level is associated with fibrosis severity in treatment-naïve, e antigen-positive patients. J Hepatol 2013;58:1089-95. [Crossref] [PubMed]
- Wang H, Ru GQ, Yan R, et al. Histologic Disease in Chinese Chronic Hepatitis B Patients With Low Viral Loads and Persistently Normal Alanine Aminotransferase Levels. J Clin Gastroenterol 2016;50:790-6. [Crossref] [PubMed]
- Wu ZQ, Tan L, Liu T, et al. Evaluation of changes of serum hepatitis B surface antigen from a different perspective. World J Gastroenterol 2015;21:2739-45. [Crossref] [PubMed]
- Ke WM, Xie SB, Yu LN, et al. Decline of serum HBV DNA and no change apportioned by the same hepatic parenchyma cell volume from hepatic fibrosis stage 1 to stage 4 during the natural history of chronic hepatitis B. Intervirology 2008;51:235-40. [Crossref] [PubMed]
- Quarleri J. Core promoter: a critical region where the hepatitis B virus makes decisions. World J Gastroenterol 2014;20:425-35. [Crossref] [PubMed]
- Moradi A, Zhand S, Ghaemi A, et al. Mutations in pre-core and basal-core promoter regions of hepatitis B virus in chronic HBV patients from Golestan, Iran. Iran J Basic Med Sci 2014;17:370-7. [PubMed]
- Weber A, Boege Y, Reisinger F, et al. Chronic liver inflammation and hepatocellular carcinoma: persistence matters. Swiss Med Wkly 2011;141:w13197 [Crossref] [PubMed]
- Zhu P, Tang Y, Wang YM. Recommendations for APASL Clinical Practice Guidelines: management of hepatitis B (updated in 2015). Journal of Clinical Hepatology 2016;32:423-8.
(English Language Editor: J. Toeh)


