
Atezolizumab compared to chemotherapy for first-line treatment in non-small cell lung cancer with high PD-L1 expression: a cost-effectiveness analysis from US and Chinese perspectives
Introduction
Lung cancer is the second most commonly diagnosed cancer and the leading cause of cancer-related deaths worldwide (1). Approximately 80% of all lung cancer cases are non-small cell lung cancer (NSCLC). A large proportion of patients are diagnosed at a metastatic stage (57%), and their 5-year relative survival rate is only 6% (2).
Prior to the introduction of immune check-point inhibitors (ICIs), platinum-based chemotherapy was the first-line treatment for metastatic NSCLC patients without epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) positive tumor mutations (3). In recent years, the emergence of ICIs has changed the treatment paradigm of metastatic NSCLC, and some ICIs, such as pembrolizumab, have already been widely used in clinical practice as a new standard of care (4).
Atezolizumab is an immunoglobulin G1 (IgG1) monoclonal antibody that binds to programmed death ligand 1 (PD-L1) on tumor cell surfaces and reactivates T cell antitumor functions by blocking interactions between PD-L1 and programmed death 1 (PD-1) (5). PD-L1 antibody was proved to induce durable tumor regression and prolonged stabilization of disease in NSCLC patients (6). The IMpower110 trial (7) demonstrated that atezolizumab treatment resulted in significantly longer OS than chemotherapy in NSCLC patients with high PD-L1 expression (PD-L1 expression on at least 50% of tumor cells or at least 10% of tumor-infiltrating immune cells as assessed), regardless of histologic type. Moreover, atezolizumab showed a manageable safety profile with a lower frequency of grade 3–4 treatment related adverse event (AE) than chemotherapy despite longer exposure. Based on this result, the US Food and Drug Administration (FDA) approved atezolizumab for first-line treatment of metastatic NSCLC with high PD-L1 expression (8). In China, the National Medical Products Administration (NMPA) approved the application of atezolizumab for first-line treatment of NSCLC on April 29, 2021 (9). These made atezolizumab the second ICI after pembrolizumab and first PD-L1 monotherapy in the treatment of high PD-L1 expression NSCLC.
Although the use of atezolizumab results in significant survival gains for NSCLC compared with chemotherapy, whether its cost reflects its potential benefit remains unknown. This study is the first economic investigation of atezolizumab for first-line treatment of NSCLC, from a US and Chinese perspective. The objective of our study was to evaluate the cost-effectiveness of atezolizumab versus platinum-based chemotherapy for first-line treatment in metastatic NSCLC with high PD-L1 expression. We present the following article in accordance with the CHEERS reporting checklist (available at https://dx.doi.org/10.21037/atm-21-4294).
Methods
We developed a partitioned survival model to evaluate the cost-effectiveness of using atezolizumab versus platinum-based chemotherapy in the first-line treatment of NSCLC. The outcomes were total cost, life-years (LYs), quality-adjusted LYs (QALYs), and incremental cost-effectiveness ratios (ICERs). A willingness-to-pay (WTP) threshold of $100,000 to $150,000 per QALY (10) and $33, 210 per QALY (3 times the gross domestic product per capita of China in 2020) were applied to the United States and China, respectively. A 3% annual discount rate was employed for both costs and outcomes (11). Statistical analysis was performed using R software (version 3.6.3, http://www.r-project.org).
Population and interventions
Medical information was derived from the IMpower110 trial (6). Patients with stage IV NSCLC who had not previously received chemotherapy were randomly assigned in a 1:1 ratio to receive either atezolizumab (1,200 mg intravenously, maximum 35 cycles) or platinum-based chemotherapy (once every 3 weeks, 4 or 6 cycles) (Table S1). Chemotherapy dosing was calculated for the model based on body surface area (United States, 1.8 m2, 70 kg; China, 1.72 m2, 65 kg) and creatinine clearance of 70 mL/minute (12,13).
According to the IMpower110 trial, the proportion of patients who received at least 1 subsequent anticancer therapy was 29.6% in the atezolizumab arm and 49.5% in the chemotherapy arm. The distribution of subsequent therapies was based on data from the IMpower110 trial; subsequent therapy details are shown in Table S2. The duration of subsequent therapy was 2.7 months, based on a real-world retrospective study (14). Following treatment, patients received best supportive care until death. A 20-year time horizon was selected to accommodate patient life expectancy.
Model structure
The partitioned survival model was developed using TreeAge Pro 2021 software (TreeAge Software, Williamstown, Massachusetts, USA) with 3 mutually independent health states: progression-free survival (PFS), progressive disease (PD), and death (Figure 1). In this model, the proportion of patients in each health state at a certain time point was calculated based on the two survival curves of PFS and OS, with the advantages of incorporating the time-varying therapeutic effect naturally and no requirements for estimating transition probabilities (15).
PFS and OS curves for atezolizumab and chemotherapy treatment were based on the results of the IMpower110 trial. Kaplan-Meier data were extracted by GetData Graph Digitizer (version 2.26; http://www.getdata-graph-digitizer.com), and time-to-event data were generated using the algorithm described in the study of Guyot et al. (16). Among the log-logistic, exponential, lognormal, generalized gamma, and Gompertz distributions, the best fit for the PFS and OS data of the atezolizumab arm was Weibull distribution, according to Akaike information criterion (AIC), Bayesian Information Criterion (BIC), and visual inspection. Log-logistic distribution fit the chemotherapy arm PFS and OS curves best, based on the same method (Figure S1).
Cost and utility estimates
Only direct medical care costs were covered in this model, including drug costs, administration costs, the cost of immunohistochemical testing, the cost of AE management, follow-up costs, best supportive care costs, and terminal care costs. Costs were estimated from US and Chinese payer perspectives. For the United States, drug costs were estimated using 2021 average sale pricing from the Centers for Medicare and Medicaid Services (17). Administration costs and follow-up costs were estimated based on the 2021 Medicare physician fee schedule (18). For China, all drug and administration costs were derived from Xiangya Hospital, Central South University and converted to US dollars with an exchange rate of $1 = RMB 6.5443.
We included severe AEs (higher than grade 3) that occurred in more than 5% of the patients in the model (Table S3). For the United States, AE costs were derived from previously published studies (19,20), and for China, they were calculated based on local charges. Best supportive care and terminal care costs were sourced from the literature (21-23). The Consumer Price Index was used to adjust costs for inflation to reflect 2021 US dollars. All information regarding costs is listed in Table 1.
Table 1
| Cost | US value | Range | Chinese value | Range | Distribution |
|---|---|---|---|---|---|
| Drug cost, US$/per cycle | |||||
| Atezolizumab | 9,382 | 7,505 to 11,258 | 5,012 | 4,009 to 6,014 | Gamma |
| Pemetrexed | 6,587 | 5,270 to 7,904 | 728.9 | 583.1 to 874.7 | Gamma |
| Carboplatin (nonsquamous NSCLC) | 30.55 | 24.44 to 36.66 | 92.61 | 74.09 to 111.1 | Gamma |
| Carboplatin (squamous NSCLC) | 25.46 | 20.37 to 30.55 | 77.18 | 61.74 to 92.61 | Gamma |
| Cisplatin | 22.69 | 18.15 to 27.23 | 15.71 | 12.57 to 18.85 | Gamma |
| Gemcitabine (with Cisplatin) | 89.28 | 71.42 to 107.14 | 1124 | 899.6 to 1,349 | Gamma |
| Gemcitabine (with Carboplatin) | 71.42 | 57.14 to 85.71 | 899.6 | 719.7 to 1,079 | Gamma |
| Nivolumab | 5,994 | 4,795 to 7,192 | 2,756 | 2,205 to 3,307 | Gamma |
| Pembrolizumab | 7,091 | 5,673 to 8,509 | 3,559 | 2,847 to 4,271 | Gamma |
| Paclitaxel | 48.51 | 38.81 to 58.21 | 967.1 | 773.7 to 1,161 | Gamma |
| Docetaxel | 97.20 | 77.76 to 116.64 | 1,281 | 1,025 to 1,537 | Gamma |
| Administration cost, US$ | |||||
| First hr | 142.55 | 122.39 to 189.18 | 0.92 | 0.73 to 1.10 | Gamma |
| Additional hr | 30.68 | 27.00 to 39.38 | 0.31 | 0.24 to 0.37 | Gamma |
| AEs cost, US$ | |||||
| Anemia | 8,779 [19] | 7023 to 10,535 | 483.7 | 387.0 to 580.4 | Gamma |
| Thrombocytopenia | 5,848 [19] | 4,678 to 7,018 | 1,811 | 1,449 to 2,173 | Gamma |
| Neutropenia | 36,346 [20] | 29,077 to 43,615 | 704.5 | 563.6 to 845.4 | Gamma |
| Immunohistochemical test | 108.38 | 96.12 to 139.05 | 152.8 | 122.24 to 183.36 | |
| Follow-up cost, US$/per month | 118.39 | 94.71 to 142.07 | 49.51 | 39.61 to 59.41 | |
| Best supportive care, US$/per month | 4,221 [21] | 3,377 to 5,065 | 485.6 [23] | 388.5 to 582.7 | Gamma |
| Cost of terminal care, US$ | 17,185 [22] | 13,748 to 20,622 | 2,205 [23] | 1,764 to 2,646 | Gamma |
NSCLC, non-small cell lung cancer; AEs, adverse events.
Utility values were applied to reflect the impact of the disease on health states and were measured by patient preference for living at a particular health state, in which 0 represents the worst health and 1 the best. The utility data used in the model (Table 2) were obtained from previously published articles (24-27).
Table 2
| Variable | Atezolizumab arm | Range | Chemotherapy arm | Range | Distribution |
|---|---|---|---|---|---|
| Incidence of AEs ( |
Beta | ||||
| Anemia | 0.017 | 0.014 to 0.020 | 0.183 | 0.146 to 0.220 | Beta |
| Thrombocytopenia | 0.003 | 0.002 to 0.004 | 0.072 | 0.058 to 0.086 | Beta |
| Neutropenia | 0.007 | 0.006 to 0.008 | 0.175 | 0.140 to 0.210 | Beta |
| Utility ( |
|||||
| Utility for PFS | 0.691 | 0.558 to 0.829 | 0.653 | 0.522 to 0.786 | Beta |
| Utility for PD | 0.473 | 0.378 to 0.568 | 0.473 | 0.378 to 0.568 | Beta |
| AEs disutility | |||||
| Anemia ( |
−0.073 | −0.058 to −0.088 | −0.073 | −0.058 to −0.088 | Beta |
| Thrombocytopenia ( |
−0.650 | −0.520 to −0.780 | −0.650 | −0.520 to −0.780 | Beta |
| Neutropenia ( |
−0.460 | −0.368 to −0.552 | −0.460 | −0.368 to −0.552 | Beta |
AEs, adverse events; PFS, progression-free survival; PD, progressive disease.
Sensitivity analysis
We conducted one-way sensitivity analysis to identify the key factors that influence cost-effectiveness. Variable ranges were obtained from the best available evidence; otherwise, a variance of ±20% of base-case values was employed. Probabilistic sensitivity analysis was conducted by running 10,000 Monte Carlo simulations to test the uncertainty of the model with all parameters simultaneously varied within a specific pattern of distribution (Tables 1,2). As proposed by the Professional Society for Health Economics and Outcomes Research (ISPOR)-Society for Medical Decision Making (SMDM) Modeling Good Research Practices Task Force (28), gamma distribution was adopted for all input costs and beta distribution was used for probability and utility estimates (29).
Results
Base-case analysis
In the base case, atezolizumab showed an additional survival benefit of 1.27 life-years (LYs) compared to chemotherapy in high PD-L1 expression NSCLC patients. Accounting for quality of life, patients in the atezolizumab group survived 0.87 QALYs longer than the chemotherapy patients (1.75 vs. 0.88 QALYs). In the United States and China, the use of atezolizumab cost an additional $107,089 and $68,489, respectively, compared with chemotherapy, yielding an ICER of $123,424/QALY and $78,936/QALY, respectively (Table 3).
Table 3
| Results | United States | China | |||||
|---|---|---|---|---|---|---|---|
| Atezolizumab | Chemotherapy | ICER | Atezolizumab | Chemotherapy | ICER | ||
| LYs | 3.14 | 1.87 | 1.27 | 3.14 | 1.87 | 1.27 | |
| QALYs | 1.75 | 0.88 | 0.87 | 1.75 | 0.88 | 0.87 | |
| Total cost, $ | 257,618 | 150,529 | 107,089 | 90,359 | 21,870 | 68,489 | |
| ICER, $/LY | 84,678 | 54,156 | |||||
| ICER, $/QALY | 123,424 | 78,936 | |||||
PD-L1, programmed death ligand 1; ICER, incremental cost-effectiveness ratio; LY, life year; QALY, quality adjusted life year.
Sensitivity analysis
Figure 2 presents the results of one-way sensitivity analysis. Figure 2A shows that for the United States, the variables with the greatest influence on the ICER were the cost of atezolizumab and utility for PFS of atezolizumab and of chemotherapy. The ICER exceeded the WTP threshold of $150,000 per QALY when the price of atezolizumab ($7.82 per milligram) increased to the upper limit ($9.38 per milligram) or the utility for PFS of atezolizumab (0.691) increased to 0.829. The ICER was lower than the WTP threshold of $100,000 per QALY when the price of atezolizumab decreased to $6.25 per milligram. For China (Figure 2B), the cost of atezolizumab, utility for PFS of atezolizumab, and the time horizon had considerable impact on the ICER. Across the variation ranges for all parameters, the ICERs remained greater than $60,000 per QALY, which was higher than the Chinese WTP threshold.
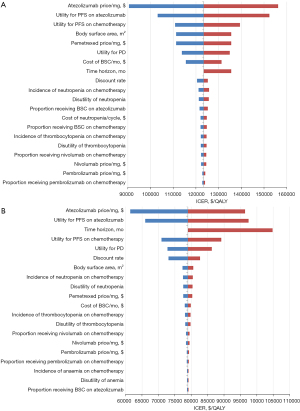
For high PD-L1 expression patients in the United States, probabilistic sensitivity analysis indicated that when the WTP was $100,000 to $150,000 per QALY, the probability of atezolizumab strategy being cost-effective compared with chemotherapy strategy was 13.1% and 85.8%, respectively (Figure 3A). For China (Figure 3B), atezolizumab was not a cost-effective option compared to chemotherapy at the WTP threshold of $33,210 per QALY. We found that when atezolizumab pricing decreased to 48% of its original cost, the ICER was below the WTP threshold.

Discussion
Almost one-quarter of all cancer deaths are due to lung cancer (18.4% of total cancer deaths), making a significant contribution to the rapidly growing global cost of cancer care (30). In recent years, ICIs have made a breakthrough in NSCLC treatment, but their high cost has placed great economic pressure on payers (31). Therefore, the association between clinical benefit and drug cost needs to be demonstrated to develop pricing strategies for immunotherapy drugs.
The phase 3 IMpower110 trial showed that atezolizumab had significant survival benefits compared with chemotherapy for metastatic NSCLC patients with high PD-L1 expression. Our study aimed to evaluate the health and economic outcomes of atezolizumab as first-line treatment of metastatic NSCLC patients, from a US and Chinese perspective.
In the United States, the base-case ICER for atezolizumab of $123,424/QALY fell within the acceptable WTP threshold range of $100,000/QALY–$150,000/QALY. Probabilistic sensitivity analysis showed that atezolizumab had a high probability (85.8%) of being determined cost-effective over chemotherapy at the upper limit of WTP threshold. Recently, a cost-effectiveness analysis of atezolizumab compared with chemotherapy from US perspective was conducted (32), demonstrating that atezolizumab cost an additional $224,590 ($311,054 vs. $86,464) and provided survival gain of 1.32 QALYs (2.36 vs. 1.08 QALYs) compared with chemotherapy, yielding an ICER of $170,730/QALY. These results have reached a conclusion that atezolizumab was estimated not to be cost-effective compared with chemotherapy for high PD-L1expression NSCLC population which is inconsistent with ours. The main reason probably lies in different models used in the two studies; partitioned survival analysis model was designed in our study, while the published article developed a Markov model and calculated survival rate of atezolizumab by multiplying the survival rate of chemotherapy and the hazard ratios (HRs), resulting in longer QALY for atezolizumab group. Different variables and costs used for models may also explain the distinction between two ICERs. Before atezolizumab was approved by the FDA, pembrolizumab monotherapy was the standard-of-care for metastatic nonsquamous NSCLC patients with PD-L1 tumor proportion score (TPS) >50%, and many studies have verified its cost-effectiveness compared to platinum-based chemotherapy in the United States (18,23,33). Our results confirmed that after pembrolizumab, atezolizumab is the second immuno-monotherapy that has been proven to be cost-effective for people with high PD-L1 expression, providing more treatment options for this population.
Until now, there has been no relevant study from a Chinese perspective for the economic evaluation of this therapeutic regimen. Contrary to the results of the United States, atezolizumab was not shown to be cost-effective compared to chemotherapy in China, which had an ICER of $78,936 per QALY, more than twice the WTP of $33,210 per QALY. The results of one-way sensitivity analysis revealed the cost of atezolizumab was the most influential parameter, indicating that a price reduction may be a feasible strategy to increase the cost-effectiveness of atezolizumab treatment. We found that when atezolizumab pricing decreased to 48% of its original cost, the ICER fell below the WTP threshold of $33,210 per QALY, which resulted in atezolizumab becoming cost-effective. This finding could help with negotiating adjustments to the cost of atezolizumab to achieve more favorable economic results. Since 2017, the Chinese National Healthcare Security Administration has conducted price negotiations for oncology drugs, with the cost of some ICIs falling significantly in order to be included on the National Reimbursement Drug List (NRDL). The Chinese government has stated that future negotiations regarding oncology drug costs will be conducted on the basis of pharmacoeconomic evaluation, and thus our results could provide evidence for any future negotiations that may occur for atezolizumab. Based on the differing results from US and Chinese perspectives, it is evident that even for the same treatment, cost-effectiveness analysis outcomes from high-income and middle-income countries might be distinct. Thus, different regions should take into account locally representative economic parameters before drug approval.
Prior to the approval of atezolizumab monotherapy for first-line treatment of NSCLC, atezolizumab in combination with chemotherapy was shown to reduce overall mortality risk compared with chemotherapy alone. Several studies evaluated the cost-effectiveness of atezolizumab combination therapy, but the results indicated that the cost was not commensurate with the survival improvements it could provide. An analysis conducted by Criss et al. revealed that compared to treatment with bevacizumab, carboplatin, and paclitaxel (BCP), the addition of atezolizumab to BCP (ABCP) was estimated to obtain an ICER of $201,676/QALY (34), while the results from another study indicated that the ICER of ABCP was $568,967/QALY and $516,114/QALY compared with BCP and carboplatin plus paclitaxel (CP), respectively (35). These results indicated that first-line treatment with ABCP was not a cost-effective treatment option for metastatic NSCLC in the United States. Cost-effectiveness analysis based on IMpower130 trial data have also shown that the addition of atezolizumab to carboplatin plus nab-paclitaxel is not an economically preferred treatment compared with chemotherapy for first-line treatment of NSCLC patients from a value standpoint in both the United States (36,37) and China (38). Although atezolizumab combination therapy has clinical benefit, it is unlikely to be cost-effective for NSCLC patients compared to chemotherapy. Our study confirmed that atezolizumab monotherapy had the advantage of having a relatively lower total cost, making it cost-effective for NSCLC patients with high PD-L1 expression in the United States. Reducing the price of atezolizumab in China would be the most effective way to make this treatment strategy economical. In addition to the clinical benefit achieved in the treatment of NSCLC, atezolizumab has made a major breakthrough in extensive-stage small-cell lung cancer (ES-SCLC). Atezolizumab or durvalumab combine with etoposide plus platinum chemotherapy (AEP and DEP respectively) significantly improve patients’ survival and were approved by FDA as a first-line options for treating ES-SCLC. In a cost-effectiveness and network meta-analysis study, AEP represented a dominant treatment strategy compared with DEP. Despite neither AEP nor DEP was cost-effective compared with EP chemotherapy, AEP was able to provide a more efficient balance between incremental cost and QALY than DEP (39). These achievements in advanced-stage lung cancer inspired investigators to conduct further clinical trials in patients with early-stage lung cancer in both adjuvant and neoadjuvant therapies (40,41), which call for corresponding cost-effectiveness research to elucidate the economic benefits of these therapies.
There were several limitations to our analysis. First, as we collected clinical information from the IMpower110 trial retrospectively, any biases within the trial could impact the outcomes of our model. Second, the median follow-up for high PD-L1 expression population in the IMpower110 clinical trial was 15.7 months, and because the model needed a time horizon long enough to simulate survival benefit and cost burden, we extrapolated the long-term PFS and OS curves using the short-term data of the clinical trial. When long-term survival data are available, we will perform a trial-based analysis to confirm or update current results. Third, we assumed that Chinese patients had the same utility values as US patients. Although this may lead to bias for the Chinese context, utility value variations were tested through sensitivity analysis, which showed that ICERs were always higher than the WTP within the settled range. Fourth, the cost of AEs of grade 1/2 or with a frequency lower than 5% in both treatment groups were excluded in this study. Some AEs, such as immune-related AEs, rarely occur but are costly to manage, which may lead to and underestimation of AE costs. Fifth, most base-case costs in China were derived from Xiangya Hospital, Central South University. Although these costs represent the price of medical care in most Chinese medical facilities, there may still be small differences across hospitals or regions, which could affect the generalizability of our findings.
Conclusions
In summary, for the United States, with a WTP threshold of $100,000 to $150,000 per QALY, atezolizumab was cost-effective for first-line treatment when compared with platinum-based chemotherapy in metastatic NSCLC patients with high PD-L1 expression. For China, atezolizumab was unlikely to be considered high-value treatment for NSCLC at a WTP threshold of $33,210 per QALY, and a price reduction of 52% appeared to be justified.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CHEERS reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-4294
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-4294). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Latest global cancer data: Cancer burden rises to 19.3 million new cases and 10.0 million cancer deaths in 2020. Date last accessed: March 2, 2021. Available online: https://www.iarc.fr/fr/news-events/latest-global-cancer-data-cancer-burden-rises-to-19-3-million-new-cases-and-10-0-million-cancer-deaths-in-2020/
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016;5:288-300. [Crossref] [PubMed]
- Doroshow DB, Sanmamed MF, Hastings K, et al. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin Cancer Res 2019;25:4592-602. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med 2020;383:1328-39. [Crossref] [PubMed]
- US Food and Drug Administration. FDA approves atezolizumab for first-line treatment of metastatic NSCLC with high PD-L1 expression. Date last accessed: March 2, 2021. Available online:https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-first-line-treatment-metastatic-nsclc-high-pd-l1-expression
- National Medical Products Administration of China. Release of information on pending receipt of drug approval certificates. Date last accessed: May 2, 2021. Available online: https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20210429162506168.html
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796-7. [Crossref] [PubMed]
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016;316:1093-103. [Crossref] [PubMed]
- Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value Health 2011;14:836-45. [Crossref] [PubMed]
- Wu B, Chen H, Shen J, et al. Cost-effectiveness of adding rh-endostatin to first-line chemotherapy in patients with advanced non-small-cell lung cancer in China. Clin Ther 2011;33:1446-55. [Crossref] [PubMed]
- Arunachalam A, Li H, Bittoni MA, et al. Real-World Treatment Patterns, Overall Survival, and Occurrence and Costs of Adverse Events Associated With Second-Line Therapies for Medicare Patients With Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer 2018;19:e783-99. [Crossref] [PubMed]
- Chouaid C, Bensimon L, Clay E, et al. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer 2019;127:44-52. [Crossref] [PubMed]
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12:9. [Crossref] [PubMed]
- Centers for Medicare and Medicaid Services. 2021 ASP Drug Pricing Files. Date last accessed: January 3, 2021. Available online: https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2021-asp-drug-pricing-files
- Centers for Medicare and Medicaid Services. License for Use of Current Procedural Terminology Fourth Edition (“CPT®”). Date last accessed: January 3, 2021. Available online: https://www.cms.gov/apps/physician-fee-schedule/search/search-results.aspx?Y=4&T=0&HT=1&CT=0&H1=96415&H2=96413&H3=96417&M=5
- Huang M, Lou Y, Pellissier J, et al. Cost Effectiveness of Pembrolizumab vs. Standard-of-Care Chemotherapy as First-Line Treatment for Metastatic NSCLC that Expresses High Levels of PD-L1 in the United States. Pharmacoeconomics 2017;35:831-44. [Crossref] [PubMed]
- Ting J, Tien Ho P, Xiang P, et al. Cost-Effectiveness and Value of Information of Erlotinib, Afatinib, and Cisplatin-Pemetrexed for First-Line Treatment of Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer in the United States. Value Health 2015;18:774-82. [Crossref] [PubMed]
- Sheehan DF, Criss SD, Chen Y, et al. Lung cancer costs by treatment strategy and phase of care among patients enrolled in Medicare. Cancer Med 2019;8:94-103. [Crossref] [PubMed]
- Huang M, Lopes GL, Insinga RP, et al. Cost-effectiveness of pembrolizumab versus chemotherapy as first-line treatment in PD-L1-positive advanced non-small-cell lung cancer in the USA. Immunotherapy 2019;11:1463-78. [Crossref] [PubMed]
- Lu S, Ye M, Ding L, et al. Cost-effectiveness of gefitinib, icotinib, and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Oncotarget 2017;8:9996-10006. [Crossref] [PubMed]
- She L, Hu H, Liao M, et al. Cost-effectiveness analysis of pembrolizumab versus chemotherapy as first-line treatment in locally advanced or metastatic non-small cell lung cancer with PD-L1 tumor proportion score 1% or greater. Lung Cancer 2019;138:88-94. [Crossref] [PubMed]
- Nafees B, Stafford M, Gavriel S, et al. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes 2008;6:84. [Crossref] [PubMed]
- Handorf EA, McElligott S, Vachani A, et al. Cost effectiveness of personalized therapy for first-line treatment of stage IV and recurrent incurable adenocarcinoma of the lung. J Oncol Pract 2012;8:267-74. [Crossref] [PubMed]
- Nafees B, Lloyd AJ, Dewilde S, et al. Health state utilities in non-small cell lung cancer: An international study. Asia Pac J Clin Oncol 2017;13:e195-203. [Crossref] [PubMed]
- Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making 2012;32:722-32. [Crossref] [PubMed]
- Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford University Press, 2006.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Verma V, Sprave T, Haque W, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer 2018;6:128. [Crossref] [PubMed]
- Peng Y, Zeng X, Peng L, et al. First-Line Atezolizumab for Metastatic NSCLC with High PD-L1 Expression: A United States-Based Cost-Effectiveness Analysis. Adv Ther 2021;38:2447-57. [Crossref] [PubMed]
- Georgieva M, da Silveira Nogueira Lima JP, Aguiar P Jr, et al. Cost-effectiveness of pembrolizumab as first-line therapy for advanced non-small cell lung cancer. Lung Cancer 2018;124:248-54. [Crossref] [PubMed]
- Criss SD, Mooradian MJ, Watson TR, et al. Cost-effectiveness of Atezolizumab Combination Therapy for First-Line Treatment of Metastatic Nonsquamous Non-Small Cell Lung Cancer in the United States. JAMA Netw Open 2019;2:e1911952 [Crossref] [PubMed]
- Wan X, Luo X, Tan C, et al. First-line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non-small cell lung cancer: A United States-based cost-effectiveness analysis. Cancer 2019;125:3526-34. [Crossref] [PubMed]
- Ding D, Hu H, Liao M, et al. Cost-Effectiveness Analysis of Atezolizumab Plus Chemotherapy in the First-Line Treatment of Metastatic Non-Squamous Non-Small Cell Lung Cancer. Adv Ther 2020;37:2116-26. [Crossref] [PubMed]
- Lin S, Luo S, Zhong L, et al. Cost-effectiveness of atezolizumab plus chemotherapy for advanced non-small-cell lung cancer. Int J Clin Pharm 2020;42:1175-83. [Crossref] [PubMed]
- Yang Z, Zhu Y, Xiang G, et al. First-line atezolizumab plus chemotherapy in advanced non-squamous non-small cell lung cancer: a cost-effectiveness analysis from China. Expert Rev Pharmacoecon Outcomes Res 2021; Epub ahead of print. [Crossref] [PubMed]
- Liu Q, Luo X, Yi L, et al. First-Line Chemo-Immunotherapy for Extensive-Stage Small-Cell Lung Cancer: A United States-Based Cost-Effectiveness Analysis. Front Oncol 2021;11:699781 [Crossref] [PubMed]
- Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786-95. [Crossref] [PubMed]
- Wakelee HA, Altorki NK, Zhou CC, et al. IMpower010: Primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer [EB/OL]. ASCO 2021, abstract. Available online: https://oncology.medicinematters.com/
(English Language Editor: A. Muijlwijk)



