Mucin1 expression in focal epidermal dysplasia of actinic keratosis
Introduction
Actinic keratoses (AKs) are generally considered as keratinocyte-derived premalignant or neoplastic skin lesions that can progress into early squamous cell carcinoma (SCC) in situ and invasive SCC which are characterized by focal epidermal dysplasia, presence of hyperkeratosis, and vascular ectasia (1-4). These lesions have been associated with cumulative exposure to ultraviolet (UV) radiations from sunlight. Particularly, UVA and UVB which in addition to producing DNA damage, inflammation, immunosuppression, oxidative stress and premature photoageing of skin, also mediate cellular responses that include keratinocyte proliferation, growth, impaired differentiation, gene expression modulation, survival and tissue remodeling (1,2,5-8). In respect to the risk of progression of AKs into SCC, some studies have estimated a rate that can vary from 0.025% to 16% for an individual lesion per year, and between 0.15% and 80% for patient with multiple AK lesions (1,5-7). The same studies have also estimated that between 20% and 26% undergo regression after 1 year. On account of this, the AKs are considered precursors of SCC with a risk of progression variable (8). However, its progression to SCC is still a matter of debate (1,4,5,7,9,10). Several histological classification systems for AK have been proposed (1,11-16). One of them, the keratinocyte intraepidermal neoplasia (KIN) system proposed by Cockerell (12) classifies the AKs based on the extension of epidermal dysplasia and risk of progression, into three histopathological stages from KIN I to KIN III, analogous to that used for histologic evaluation of HPV-associated cervical intraepithelial neoplasia (CIN) (12-14). The histopathological features of KIN I include foci of atypical basal keratinocytes confined to the lower third of the epidermis. In KIN II, atypical keratinocytes appear limited to the lower two thirds of the epidermis, whereas in KIN III the atypical keratinocytes extend throughout the full thickness of the epidermis; this latter degree is regarded as a SCC in situ (12-14). In the distinction between AK and SCC, several immunohistochemical studies have provided evidence supporting the transition of AK to SCC (1,5-7,12,17-20). However, the exact sequence of cellular events that occur during the progression of AK remains to be elucidated. A transmembrane glycoprotein that contributes to the progression of certain premalignant and malignant lesions is mucin1 (MUC1) (21-23). MUC1, also known as DF 3, CA 15-3, EMA or episialin, is considered as a molecular sensor of the cell surface and local environment; and a signal transducer that responds to external stimuli generating cellular responses which include loss of cell polarization, proliferation, growth, adhesion, differentiation, migration, invasion, survival and secretion of growth factors and cytokines (21,22,24,25). In human skin, MUC1 is not expressed by the normal epidermis, but is expressed by keratinocytes in premalignant lesions such as Epidermolysis bullosa and malignant lesions including Paget’s disease, Bowen’s disease, and Merkel’s carcinoma (26-29). Nevertheless, the functions of MUC1 in the skin lesions are not yet fully clear. Therefore, we investigated whether MUC1 is present in the focal epidermal dysplasia of the different histopathological stages of AK. We also sought to determine whether the expression of MUC1 could be associated to the extension of epidermal dysplasia and the risk of progression.
Methods
Skin biopsies
Fourteen skin biopsies from patients diagnosed clinically and histopathologically with AK were retrieved from the archives of the Section of Dermatopathology of SAIB. Their characteristics appear summarized in Table 1. They were classified according to the degree of epidermal dysplasia and risk of malignant transformation into SCC in KIN I, KIN II, and KIN III degrees (12,13). In five biopsies the three degrees of dysplasia were present, in two biopsies both KIN I and KIN II, in four biopsies only KIN I, and in three biopsies only KIN III. They were taken from five males and nine females, ranging in age from 38 to 80 years old, with an average of 61 years old. Five normal skin specimens were obtained from patients undergoing aesthetic surgery procedures. This study was performed according to the Declaration of Helsinky.
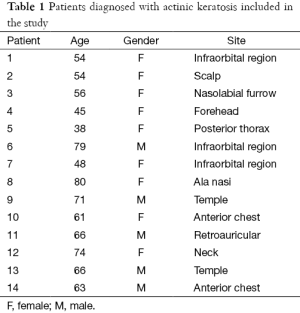
Full table
Indirect immunofluorescence staining
Paraffin-embedded skin tissue blocks were cut in sections of 4 µm of thick. Paraffin sections were placed on silanized slides (DakoCytomation, Denmark), deparaffinized in xylene, dehydrated through graded alcohol series (from 100% to 70%), and then placed in distilled water and equilibrated in phosphate-buffered saline (PBS) for 10 min. Non-specific antibody staining was blocked by incubating sections in PBS containing 2% bovine serum albumin (BSA) and 0.1% Tween 20 for 1 h at room temperature (RT). The sections were incubated overnight at 4 °C in a humid chamber with mouse monoclonal anti-human MUC1 (clone BC2) antibody (Santa Cruz Biotechnology Inc. Santa Cruz, CA). Normal intestine and dermal appendages were used as negative and positive internal control respectively, for MUC1. Samples were washed in PBS and incubated for 30 min with anti-mouse Alexa Fluor 488 (Molecular Probes, Life Technologies, USA) at RT, followed by 15 min incubation with DAPI (4’,6-Diamidino-2-phenylindole) (Molecular Probes, Life Technologies, USA) for nuclear staining. Finally, the sections were washed in PBS and coverslipped with mounting medium (IMMU-MOUNT, Shandon, Pittsburg, PA).
For immunodetection of CD44, sections were incubated with a mouse monoclonal anti-human CD44 (clone DF1485) (DakoCytomation). All images were captured using a 1X81 Olympus inverted microscope with the Fluo View Confocal Laser Scanning configuration (CLSM) (Olympus America) equipped with software program FV10.ASW version 02.01.01.04 (Olympus Corporation). Image J software (NHI, Washington DC) was used for processing of contrast and brightness. Full thickness of non-keratinized epidermis and thickness of focal epidermal dysplasia were measured on CLSM captured images in one biopsy that exhibited the three degrees of dysplasia. Eight fields were measured by each degree of dysplasia. They were defined as the distance between the basal surface of basal keratinocytes and the apical surface of granular keratinocytes. The student’s test (t) was used for comparisons between full thickness of non-keratinized epidermis and thickness of focal epidermal dysplasia.
Results
Histopathological analysis of the AK biopsies showed areas of hyperkeratosis and focal epidermal dysplasia with atypical keratinocytes characterized by loss of polarity, variation in size and shape and prominent, irregular, and hyperchromatic nuclei (Figure 1A). In some areas, these atypical keratinocytes were organized as cohesive multicellular groups that appeared to maintain together by means of intercellular bridges, possibly changing position due to an expansive growth of these cells, a mode of cell movement (30-33) (Figure 1B). Additionally, some modifications in the dermis such as solar elastosis, alterations of collagen fibers, vascular ectasia and presence of inflammatory infiltrate were also observed (Figure 1A).
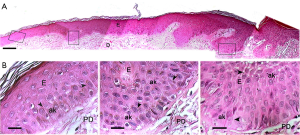
In vivo presence of MUC1
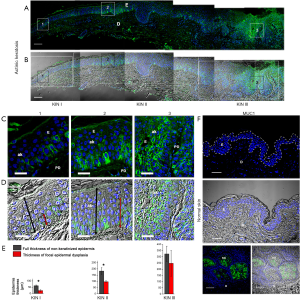
To determine whether MUC1 was present in the focal dysplasia observed in the three histopathological stages of the AKs, deparaffinized sections were examined by immunofluorescence staining. Immunostaining analyzed by CLSM revealed that MUC1 was present over the entire cell surface of only a few atypical basal keratinocytes that were confined to the lower third of the epidermis (KIN I) (Figure 2A-E). While in KIN II where the atypical keratinocytes were organized as cohesive groups and occupying the lower two thirds of the epidermis, MUC1 in addition to being localized at the apical surface of some atypical keratinocytes, was also localized over the entire cell surface of some of them (Figure 2A-E). Interestingly, in KIN III where the atypical keratinocytes extend throughout the full thickness of the epidermis, the presence of MUC1 was noticeable in the apical surface and over the entire cell surface on many of these cells (Figure 2A-E). Conversely, there was no expression of MUC1 in the epidermis of normal adult human skin (Figure 2F), since it was limited only to dermal appendages, particularly to sebaceous and sweat glands as expected (Figure 2F). No expression of MUC1 was detected when normal human intestine was used as negative control (34) (not shown).
In vivo CD44 immunolocalization
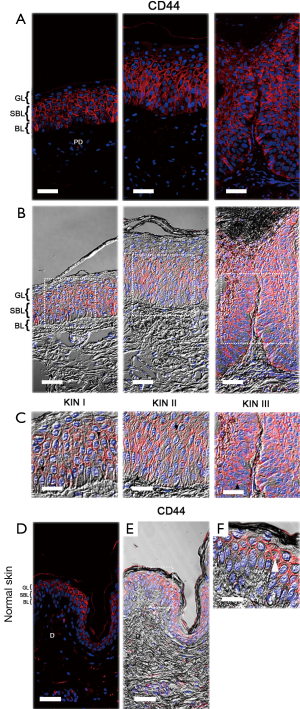
CD44, the main hyaluronan (HA) receptor, is a cell-surface glycoprotein that has been considered as an early marker for malignant transformation and as prerequisite for tumor progression (35,36). It is also involved in many cellular processes which include growth, proliferation, differentiation, cell-cell and cell-matrix adhesions, survival, motility and migration (37-40); therefore, we also examined the distribution and localization of CD44 in the epidermis of the AKs selected.
Examination of epidermis in KIN I and KIN II degrees revealed that CD44 was localized in the basal and suprabasal layers and not restricted to the areas of focal dysplasia as MUC1, whereas in KIN III, it appeared in full thickness of the epidermis (Figure 3A,B). CD44 was particularly found outlining the cellular margins and intercellular bridges of the basal and suprabasal keratinocytes (Figure 3C). In contrast, in normal epidermis CD44 was concentrated in the uppermost cell layers of the epidermis decreasing through suprabasal layers (Figure 3D-F).
Discussion
In the current study, we found that MUC1 is present in the focal epidermal dysplasia of AK. MUC1 was found at the apical surface of some atypical keratinocytes, while in other cells it was detected over the entire cell surface, organized as cohesive multicellular groups, possibly changing position due to an expansive growth of these cells (30-33). It is important to highlight that in the epidermis samples from healthy patients, MUC1 was not evidenced. Remarkably, previous studies in human skin have shown that MUC1 is not expressed by normal epidermal cells but can be expressed by keratinocytes in premalignant lesions such as Epidermolysis bullosa and malignant lesions that include Paget’s disease, Bowen’s disease, and Merkel’s carcinoma (26-29). However, as the same studies indicated, MUC1 functions in the skin lesions are not yet fully clear. In this respect, some previous in vivo and in vitro studies in normal and transformed epithelial cells have showed that MUC1 is usually localized at the apical surface of most normal secretory epithelial cells and that when cells lose its polarity, it appears on the entire surface of transformed cells (21,22,24,25,41-43). Interestingly, the same studies suggest that MUC1 overexpression reduces cell-cell and cell-extracellular matrix interactions, therefore promoting epithelial cell motility and migration (21,22,24,25). Similarly, recent studies related to MUC1 expression and function in metastatic cancers have suggested that MUC1 through its extracellular domain (also MUC1-N terminal subunit) promotes cell migration and invasion (22,25,43,44). Yet, the role of the MUC1-N in cancer is unclear (22,23,25,44). Regarding the possible role of MUC1 during wound healing and malignant conditions, some studies highlighted that MUC1 contributes to loss of cell polarity, altered proliferation, adhesion, differentiation, motility, migration, survival, and secretion of growth factors and cytokines (21,22,24,25,41,42). Moreover, loss of polarity and partial to complete alteration of the differentiation program of keratinocytes have been recently proposed to occur during the progression of AK to SCC (1,2). Therefore, we believe that the expression of MUC1 in AK would be induced by loss of polarization and alteration of the stratification and differentiation of the keratinocytes, probably occasioned by certain predisposing factors for its progression to SCC such as excessive exposure to UVR (1,2,5-8). Once positioned over the membrane, MUC1 might facilitate the motility and expansive growth of atypical keratinocytes. In this study, we also found that the presence of MUC1 change with the extension of dysplasia. In fact, in KIN III in which the atypical keratinocytes extend through the full thickness of the epidermis, the expression of MUC1 was higher than in both KIN I and KIN II (Figure 4). Accordingly, our findings suggest that the expression of MUC1 in AK would be associated to the degree of dysplasia rather than the thickness of the epidermis (Figure 2D,E).
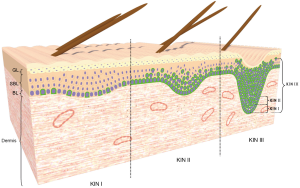
In addition to these findings, we also found alterations in CD44 distribution in the epidermis of AKs. CD44 was restricted to the basal and suprabasal layers in KIN I and KIN II, whereas in KIN III it appeared throughout the epidermis. In contrast, in normal epidermis CD44 was concentrated in the uppermost cell layers decreasing through suprabasal layers. We believe that such changes may be associated to alterations in the stratification and differentiation of keratinocytes during the transformation of AK into SCC. Consistent with this, studies in experimental animal models have provided evidence that prolonged exposure to UVR produces alterations in the expression of CD44 and accumulation of HA, suggesting that these changes are associated with the development of epidermal hyperplasia and that they may be considered as early indicators of malignant transformation and prerequisite for tumor development (35,36). In this context, some studies have shown that CD44 influences keratinocyte proliferation and differentiation (37-40). Of significance, expression of CD44 in human fetal skin has been associated with stratification and differentiation of keratinocytes (45). In relation to the localization of CD44 in AK outlining the cellular margins and in the intercellular bridges of the basal and suprabasal keratinocytes, evidence suggest that in tumor cells the levels of expression and localization of CD44 not only would contribute to the cell movement, but also determine whether the movement of the cells as multicellular groups occurs passively (expansive growth) or collectively (collective cell migration) where the intercellular bridges are not disrupted completely (31,46,47).
Thus, it is possible to hypothesize that the focal epidermal dysplasia observed during the transition of AK in addition to involve the loss of polarity and alterations of the stratification and differentiation of keratinocytes, also would involve the expansive growth of these cells where MUC1 and CD44 seem to play a crucial role.
Acknowledgements
We thank to Arch. Rachel Arciniegas M. for performing the scheme.
This work was supported by Servicio Autónomo Instituto de Biomedicina (SAIB).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ratushny V, Gober MD, Hick R, et al. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest 2012;122:464-72. [PubMed]
- Berman B, Cockerell CJ. Pathobiology of actinic keratosis: ultraviolet-dependent keratinocyte proliferation. J Am Acad Dermatol 2013;68:S10-9. [PubMed]
- Werner RN, Sammain A, Erdmann R, et al. The natural history of actinic keratosis: a systematic review. Br J Dermatol 2013;169:502-18. [PubMed]
- Fernández-Figueras MT, Carrato C, Sáenz X, et al. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J Eur Acad Dermatol Venereol 2015;29:991-7. [PubMed]
- Feldman SR, Fleischer AB Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis 2011;87:201-7. [PubMed]
- Torezan LA, Festa-Neto C. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol 2013;88:775-86. [PubMed]
- Dodds A, Chia A, Shumack S. Actinic keratosis: rationale and management. Dermatol Ther (Heidelb) 2014;4:11-31. [PubMed]
- Goldenberg G, Perl M. Actinic keratosis: update on field therapy. J Clin Aesthet Dermatol 2014;7:28-31. [PubMed]
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol 2006;155:9-22. [PubMed]
- Wells JW. Do actinic keratoses and superficial squamous cell carcinomas have a specific immunoprofile? Curr Probl Dermatol 2015;46:36-41. [PubMed]
- Hashimoto K, Mehregan A. Actinic keratosis. In: Tumors of the epidermis. Boston: Butterworth Publisher, 1990;168-6.
- Cockerell CJ. Histopathology of incipient intraepidermal squamous cell carcinoma ("actinic keratosis"). J Am Acad Dermatol 2000;42:11-7. [PubMed]
- Fu W, Cockerell CJ. The actinic (solar) keratosis: a 21st-century perspective. Arch Dermatol. 2003;139:66-70. [PubMed]
- Anwar J, Wrone DA, Kimyai-Asadi A, et al. The development of actinic keratosis into invasive squamous cell carcinoma: evidence and evolving classification schemes. Clin Dermatol 2004;22:189-96. [PubMed]
- Cockerell CJ, Wharton JR. New histopathological classification of actinic keratosis (incipient intraepidermal squamous cell carcinoma). J Drugs Dermatol 2005;4:462-7. [PubMed]
- Röwert-Huber J, Patel MJ, Forschner T, et al. Actinic keratosis is an early in situ squamous cell carcinoma: a proposal for reclassification. Br J Dermatol 2007;156 Suppl 3:8-12. [PubMed]
- Krunic AL, Garrod DR, Madani S, et al. Immunohistochemical staining for desmogleins 1 and 2 in keratinocytic neoplasms with squamous phenotype: actinic keratosis, keratoacanthoma and squamous cell carcinoma of the skin. Br J Cancer 1998;77:1275-9. [PubMed]
- Lentini M, Schepis C, Cuppari DA, et al. Tenascin expression in actinic keratosis. J Cutan Pathol 2006;33:716-20. [PubMed]
- Szeimies RM, Torezan L, Niwa A, et al. Clinical, histopathological and immunohistochemical assessment of human skin field cancerization before and after photodynamic therapy. Br J Dermatol 2012;167:150-9. [PubMed]
- Rigel DS, Stein Gold LF. The importance of early diagnosis and treatment of actinic keratosis. J Am Acad Dermatol 2013;68:S20-7. [PubMed]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 2004;4:45-60. [PubMed]
- Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 2009;9:874-85. [PubMed]
- Horm TM, Schroeder JA. MUC1 and metastatic cancer: expression, function and therapeutic targeting. Cell Adh Migr 2013;7:187-98. [PubMed]
- Zhao Q, Piyush T, Chen C, et al. MUC1 extracellular domain confers resistance of epithelial cancer cells to anoikis. Cell Death Dis 2014;5:e1438. [PubMed]
- Liu X, Yi C, Wen Y, et al. Interactions between MUC1 and p120 catenin regulate dynamic features of cell adhesion, motility, and metastasis. Cancer Res 2014;74:1609-20. [PubMed]
- Cooper HL, Cook IS, Theaker JM, et al. Expression and glycosylation of MUC1 in epidermolysis bullosa-associated and sporadic cutaneous squamous cell carcinomas. Br J Dermatol 2004;151:540-5. [PubMed]
- Yoshii N, Kitajima S, Yonezawa S, et al. Expression of mucin core proteins in extramammary Paget's disease. Pathol Int 2002;52:390-9. [PubMed]
- Kurzen H, Kaul S, Egner U, et al. Expression of MUC 1 and Ep-CAM in Merkel cell carcinomas: implications for immunotherapy. Arch Dermatol Res 2003;295:146-54. [PubMed]
- Chakraborty S, Bonthu N, Swanson BJ, et al. Role of mucins in the skin during benign and malignant conditions. Cancer Lett 2011;301:127-41. [PubMed]
- Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol 2010;188:11-9. [PubMed]
- Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 2011;147:992-1009. [PubMed]
- Friedl P, Locker J, Sahai E, et al. Classifying collective cancer cell invasion. Nat Cell Biol 2012;14:777-83. [PubMed]
- Rørth P. Fellow travellers: emergent properties of collective cell migration. EMBO Rep 2012;13:984-91. [PubMed]
- Lindén SK, Florin TH, McGuckin MA. Mucin dynamics in intestinal bacterial infection. PLoS One 2008;3:e3952. [PubMed]
- Siiskonen H, Törrönen K, Kumlin T, et al. Chronic UVR causes increased immunostaining of CD44 and accumulation of hyaluronan in mouse epidermis. J Histochem Cytochem 2011;59:908-17. [PubMed]
- Orian-Rousseau V, Ponta H. Perspectives of CD44 targeting therapies. Arch Toxicol 2015;89:3-14. [PubMed]
- Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003;4:33-45. [PubMed]
- Pasonen-Seppänen S, Hyttinen JM, Rilla K, et al. Role of CD44 in the organization of keratinocyte pericellular hyaluronan. Histochem Cell Biol 2012;137:107-20. [PubMed]
- Orian-Rousseau V, Sleeman J. CD44 is a multidomain signaling platform that integrates extracellular matrix cues with growth factor and cytokine signals. Adv Cancer Res 2014;123:231-54. [PubMed]
- Bourguignon LY, Bikle D. Selective Hyaluronan-CD44 Signaling Promotes miRNA-21 Expression and Interacts with Vitamin D Function during Cutaneous Squamous Cell Carcinomas Progression Following UV Irradiation. Front Immunol 2015;6:224. [PubMed]
- Ligtenberg MJ, Buijs F, Vos HL, et al. Suppression of cellular aggregation by high levels of episialin. Cancer Res 1992;52:2318-24. [PubMed]
- Wesseling J, van der Valk SW, Vos HL, et al. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol 1995;129:255-65. [PubMed]
- Baldus SE, Engelmann K, Hanisch FG. MUC1 and the MUCs: a family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci 2004;41:189-231. [PubMed]
- Syrkina MS, Rubtsov MA, Potashnikova DM, et al. Cell Models for the Investigation of the Role of the Mucin MUC1 Extracellular Domain in Metastasizing. Acta Naturae 2014;6:62-70. [PubMed]
- Tuhkanen AL, Agren UM, Tammi MI, et al. CD44 expression marks the onset of keratinocyte stratification and mesenchymal maturation into fibrous dermis in fetal human skin. J Histochem Cytochem 1999;47:1617-24. [PubMed]
- Schmidt S, Friedl P. Interstitial cell migration: integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res 2010;339:83-92. [PubMed]
- Gritsenko PG, Ilina O, Friedl P. Interstitial guidance of cancer invasion. J Pathol 2012;226:185-99. [PubMed]


