Characterization of acute respiratory infections among 340 infants in Wuxi, Jiangsu Province
Introduction
Human respiratory viruses are a diverse group of pathogens that invade the respiratory tract and cause the damage of tissues and organs outside the respiratory tract. Respiratory tract infection is one of the most common disease in children. It has aroused widespread attention as an important cause of death in children (1). Viral etiology of respiratory tract diseases varies with different countries, district, seasons and ages. As important pathogen in respiratory infection, common respiratory viruses includes influenza virus A (FA), influenza virus B (FB), parainfluenza virus I (PIVI), parainfluenza virus II (PIVII), parainfluenza virus III (PIVIII), adenovirus (ADV), and respiratory syncytial virus (RSV) were detected using direct immunofluorescence method. In our current study, we characterized the seven common respiratory viruses in Wuxi and analyzed their distribution in terms of gender, age, and season.
Materials and methods
Materials
A total of 340 pediatric patients (201 males and 139 females aged 1 month to 14 years) who were treated/admitted in Wuxi Second People’s Hospital from June 2012 to May 2014 were enrolled in this study. The diagnosed diseases included bronchitis, bronchiolitis, pneumonia, asthmatic bronchitis, and upper respiratory tract infections.
Methods
Main reagents
D3(R) Ultra(TM) DFA Respiratory Virus Screening and Identification Kit for Seven Major Respiratory Viruses (Diagnostic Hybrids, USA) was applied for the detection of the monoclonal antibodies of seven respiratory viruses including FA, FB, PVI, PVII, PVIII, ADV and RSV. Other materials included porous slides, positive quality control plates, mounting medium, self-prepared PBS buffer, acetone, and glycerine.
Main instruments
Inverted fluorescence microscope (OLYMPUS IX51), tabletop centrifuge (THERMO S-16R), vortex mixer (Shanghai Huxi XW-80A), 37 °C electric heating constant temperature incubator (Shanghai Jinghong DK-600), and biosafety cabinet (Suzhou Antai BSC-130011B2).
Sample collection
Nasopharyngeal swab specimens were collected under sterile conditions by assigned medical staff on the day of admission or the next morning, during which the plastic tube was inserted 7-8 cm into the pharynx via nasal cavity to suction 1-2 mL of secretions. The specimens were immediately placed into a 15-mL sterile centrifuge tube and then sent to the clinical laboratory.
Preparation of cell smears
The nasopharyngeal secretions were broken and mixed well in a vortex oscillator and then centrifuged at 2,000 rpm. Discard the supernatant and wash the sediment twice with PBS. Load it on a porous slide and air dries it at room temperature. After the sediment was fixed in acetone at 2-6 °C, seven fluorescein-labeled monoclonal antibodies to antigens were added for fluorescence microscopy.
Result judgment
Fluorescence microscopy (200×) showed apple-green fluorescence inside respiratory virus antigen-positive cells and red fluorescence inside negative cells. Fluorescence images can refer to positive quality control plates. Viral infection was judged to be positive if >2 green fluorescence cells were found in each visual field under inverted fluorescence microscope.
Statistical analysis
Statistical analysis was performed using SPSS 13.0 software. Comparison of rates was performed using chi square test, with a P value of less than 0.05 being considered statistically significant.
Results
Detection results of seven respiratory viruses
Of these 340 patients, 116 (34.12%) were found to be virus-positive. Among these seven respiratory viruses, RSV had the highest positive rate (16.18%; n=55), followed by ADV (3.82%; n=13), FA (5.00%; n=17), FB (5.29%; n=18), PIVI (1.47%; n=5), PIVII (1.76%; n=6), and PIVIII (0.59%; n=2) (Table 1).
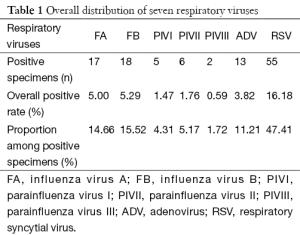
Full table
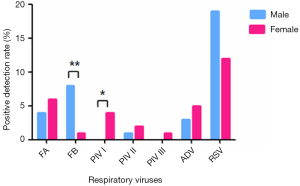
Distribution of respiratory viruses in males and females
The positive rate of respiratory viruses was 36.32% (73/201) in males and 31.65% (44/139) in females (χ2=0.79, P>0.05). The positive rates of each respiratory virus in males and females are shown in Figure 1. Notably, the positive rates of FB and PIVI were significantly different between males and females (χ2=6.97, P<0.01; χ2=5.07, 0.01<P<0.05).
Detection results of seven respiratory viruses in different age groups
Among the three age groups, the 0-1-year-old group had the highest positive rate (48.48%; 32/66), followed by the 3-14-year-old group (31.61%; 49/155) and 1-3-year-old group (29.41%; 35/119) (P<0.05). The 0-1-year-old group and 1-3-year-old group had the highest positive rates of RSV [36.37% (24/66) and 19.33% (23/119), respectively]. In contrast, the 3-14-year-old group had the highest positive rate of FB (10.32%; 16/155) (Table 2).
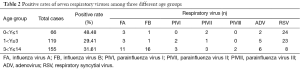
Full table
Seasonal distribution of respiratory virus infections
According to the astronomical seasons in the northern hemisphere and the actual weather in Wuxi, we divided March, April, and May as spring, June, July, and August as summer, September, October, and November as autumn, and December, January and February as winter (2). In Wuxi, respiratory virus infections were mainly detected in winter (42.72%; 44/103), followed by spring (38.32%; 44/107), autumn (29.11%; 23/79) and summer (15.69%; 8/51) (χ2=12.82, P<0.01). Among these seven respiratory viruses, RSV had the highest detection rates in spring, autumn, and winter, whereas FB was the most commonly detected virus in summer. PIVIII had the lowest detection rates in all four seasons (Table 3).
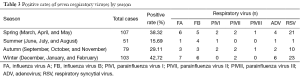
Full table
Discussion
According to the World Health Organization, about 1.9 million children worldwide die each year from respiratory tract infection and its complications (3). Respiratory tract infection has been listed as the second leading cause of death in children under five (4). Viruses are key pathogens of respiratory infections (5,6). Characterization of respiratory viruses and understanding its relationships with gender, age, and season will help to carry out the prevention and treatment of childhood respiratory tract infections and indirectly reduce the antibiotic abuse in clinical settings.
The overall detection rates of viruses dramatically vary among different areas. In our current study, the positive rate of viruses was 34.12%, which may be mainly due to the variations in different geographical environments and climatic characteristics. It has also been reported that the overall detection rates of viruses are correlated with the monthly mean air temperature and wind speed (7). Also, the differences in virus profiles in different areas may also contribute to the difference in the overall detection rate. Finally, different virus detection methods (e.g., molecular biological techniques and immunofluorescence techniques) may also lead to the difference in virus detection rate.
In our current study, RSV is the most commonly detected respiratory virus, suggesting that this virus is the leading pathogen of childhood respiratory tract infections in Wuxi. This result is consistent with the findings in most studies conducted in China and abroad (8-11). In our study, RSV had the highest detection rate in spring, autumn, and winter. Currently, RST is the well recognized pathogen of respiratory tract infections among young children. For instance, the data in Kunming (12) showed that the RSV-positive specimens accounted for up to 83.3% of all positive specimens, which are consistent with findings in Beijing and Chongqing. Similar findings have also seen in the United States (13), Brasil (14), and the United Kingdom (15). However, some domestic studies have also reported that the leading pathogen was influenza viruses (16). In our study, FB had the highest detection rate in summer, which may be explained by the differences in detection methods, geographical factors, and weather. In our study, the overall positive rate of viruses was not significantly different between males and females, indicating that there is no sexual dimorphism in susceptibility to respiratory virus infections. We also found the respiratory viruses had higher positive rates in 0-1-year-old group, which may because younger children have weaker resistance and resilience to viral infections; compared with the 1-3-year-old group, children in the 3-14-year-old group had an increased positive rate of respiratory virus infection, which may be explained as follows: children in this age group have entered their school-age; although their immunity has enhanced, the accumulation and exchanges among children in schools facilitate viral spread. Therefore, prevention and control of respiratory viruses should be a priority for newly enrolled children. Many respiratory viruses have characteristic seasonal patterns (17). In our current study, the season with the highest incidences of respiratory virus infections was winter, followed by spring. The possible reason may as follows: the city of Wuxi is located in the Yangtze River Delta where there are many rivers with high humidity. In particular, the weather is cold and humid in winter. Even worse, there is big temperature difference between indoor and outdoor experience, and the indoor ventilation is often poor. As a result, the respiratory virus infections are frequent in this season. Therefore, seasonal prevention is particularly important for these conditions.
Qian et al. (18) surveyed the epidemiology of hospitalized pediatric patients in Wuxi Children’s Hospital from 2008 to 2009 and found that the main viral pathogen was RSV, in particular in winter and spring; the positive rate was significantly higher in children under 3 years than in those older than 3 years. Their findings were consistent with ours, indicating the epidemiology of respiratory viruses remains stable in Xuxi in the past 5 years. The overall viral infection showed no significant difference between males and females. In contrast, Qian et al. (18) found that it was significantly higher in males than in females (P<0.01), which might be explained by the difference in the research populations: Qian et al.’s study was focused on a large sample of hospitalized pediatric patients, whereas our study enrolled both outpatients and inpatients, and the sample size was relatively small.
Our study was limited by the fact that it was conducted in a single center in Wuxi; in future, multi-center studies with larger sample sizes should be performed. Due to the lack of specific clinical manifestations of respiratory tract diseases caused by various viruses, it is particularly important to learn the epidemiological features of respiratory virus infections in local areas. Our study characterized the pathogens that cause childhood respiratory tract infections in Wuxi area and these data may inform the prevention and treatment of clinical diseases in this area.
Acknowledgements
Funding: This work was supported by the Projects of Health Department in Wuxi (FYKY201409).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Heikkinen T, Silvennoinen H, Peltola V, et al. Burden of influenza in children in the community. J Infect Dis 2004;190:1369-73. [PubMed]
- Lv XJ, Xu D, Chen ZM. Epidemiological characteristics of viral respiratory tract infections in children in Hangzhou. Zhonghua Liu Xing Bing Xue Za Zhi 2008;29:846-7. [PubMed]
- Mulholland K. Global burden of acute respiratory infections in children: implications for interventions. Pediatr Pulmonol 2003;36:469-74. [PubMed]
- Bryce J, Boschi-Pinto C, Shibuya K, et al. WHO estimates of the causes of death in children. Lancet 2005;365:1147-52. [PubMed]
- Khamrin P, Thongprachum A, Shimizu H, et al. Detection of human bocavirus 1 and 2 from children with acute gastroenteritis in Japan. J Med Virol 2012;84:901-5. [PubMed]
- Moriyama Y, Hamada H, Okada M, et al. Distinctive clinical features of human bocavirus in children younger than 2 years. Eur J Pediatr 2010;169:1087-92. [PubMed]
- Dijkman R, Koekkoek SM, Molenkamp R, et al. Human bocavirus can be cultured in differentiated human airway epithelial cells. J Virol 2009;83:7739-48. [PubMed]
- Jia L. Detection of viruses causing lower respiratory tract infection in children. Chinese Journal of Nosocomiology 2011;21:3386-8.
- Bezerra PG, Britto MC, Correia JB, et al. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS One 2011;6:e18928. [PubMed]
- Kim MR, Lee HR, Lee GM. Epidemiology of acute viral respiratory tract infections in Korean children. J Infect 2000;41:152-8. [PubMed]
- Lin TY, Huang YC, Ning HC, et al. Surveillance of respiratory viral infections among pediatric outpatients in northern Taiwan. J Clin Virol 2004;30:81-5. [PubMed]
- Wu X, Ni L, Xian FM, et al. Clinical and Epidemiological Characteristics Of Pathogenic Analysis in children with acute lower respiratory infection. Contemporary Medicine 2010;16:57-8.
- Murata Y. Respiratory syncytial virus infection in adults. Curr Opin Pulm Med 2008;14:235-40. [PubMed]
- Bellei N, Carraro E, Perosa A, et al. Acute respiratory infection and influenza-like illness viral etiologies in Brazilian adults. J Med Virol 2008;80:1824-7. [PubMed]
- Lees EA, Carrol ED, Gerrard C, et al. Characterisation of acute respiratory infections at a United Kingdom paediatric teaching hospital: observational study assessing the impact of influenza A (2009 pdmH1N1) on predominant viral pathogens. BMC Infect Dis 2014;14:343. [PubMed]
- Zhou WH, Kang WQ. Analysis of pathogeny of virus infection in children's respiratory track. Henan J Prev Med 2007;18:412-4.
- Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health 1998;3:268-80. [PubMed]
- Qian J, Wang WJ, Xie JJ, et al. A study on viral pathogen of acute respiratory infection in 654 hospitalized children. Jiangsu Med J 2008;34:569-70.