Membranous glomerulonephritis and cellular crescents induced by levamisole-adulterated cocaine abuse: a case report
Introduction
Levamisole is a synthetic imidazothiazole derivative used for its immunomodulatory properties. In fact, during the 80s and 90s, levamisole was used as an immunomodulatory agent for the treatment of a variety of autoimmune conditions and malignancies (1,2). Nowadays it is exclusively licensed as an antihelminthic for use in veterinary medicine. However, it is also illicitly employed as a cocaine adulterant (3). It has recently been reported that the consumption of levamisole-adulterated cocaine can provoke anti-neutrophil cytoplasmic antibody (ANCA)-associated syndromes. Additionally, levamisole causes loss of tolerance to several antigens from within neutrophil granules, eventually resulting in clinical disease (4,5).
Interestingly, genetic and environmental factors likely play an important role (6). Patients carrying an HLAB27 allele are known to be at higher risk of developing agranulocytosis when treated with levamisole (7). Likewise, patients with ANCA-associated vasculitis (AAV) and internal organ involvement have typically been exposed to offending agents for prolonged periods of time, often on the order of years. This phenomenon is likely true in those exposed to levamisole, given the widespread use of cocaine, the high prevalence of it in the drug supply, and the relatively low incidence of their manifestations (6).
Here, we report an unusual case of a patient with a membranous glomerulonephritis (GNM) and cellular crescent associated with levamisole-contaminated cocaine use.
Case report
The patient was a 49-year-old Caucasian man who was a regular user of intravenous drugs, was infected with the hepatitis C virus (HCV), with fibroscan and no sustained elevation of transaminases, was diagnosed with microcytic anemia and had purple skin with ANCA-positive pseudovasculitis from levamisole use (a skin biopsy was performed in 2010). The man was admitted to the Division of Nephrology with acute oligoanuric renal failure and macroscopic hematuria, and with the appearance of new skin-necrotic lesions on his nose, trunk and extremities. The patient confirmed that the last parenteral cocaine consumption had been 3-4 days before. In additional tests, renal failure was highlighted with serum creatinine levels of 7.09 mg/dL, 263 mg/dL for urea and 5.5 mEq/L for potassium. Elevated serum acute phase reactants were also observed, such as C-reactive protein (CRP): 212.2 mg/L; procalcitonin: 20.43 ng/mL; and albumin: 2 g/dL (without edema), as well as methicillin-resistant Staphylococcus aureus in serial blood cultures. Prothrombotic elevation parameters were also recorded, giving a D-dimer value of 5,825 ng/mL and anemia with hemolytic characteristics [hemoglobin (Hb) concentration: 8.9 g/dL; mean corpuscular volume: 76 fL; platelet count: 65×103/μL; and LDH: 766 U/L]. It is also worth mentioning the levels of serum immunological parameters: 52 mg/dL for C3; 7 mg/dL for C4; ANCA antibodies were positive with a perinuclear staining pattern (1:1,280); myeloperoxidase (MPO)-ANCA and proteinase 3 (PR3)-ANCA antibodies were positive (56.4 and 47.10 U/mL, respectively), and anti-glomerular basement membrane antibodies were negative (7 U/mL). After recovering diuresis, a proteinuria of 1.35 g/24 h was reported whereas dysmorphic erythrocytes and cell casts were also observed in the urinary sediment.
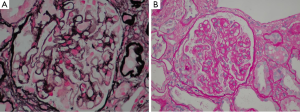
An abdominal ultrasound showed kidneys with normal cortico-medullary differentiation and a minimal amount of free fluid in the pelvis with no other pathological findings. The transthoracic echocardiography (TTE) was negative for endocarditis. Physical examination revealed tense ascites of unknown origin whilst the subsequent peritoneal biopsy showed vasculopathy (peritoneal thrombotic vasculitis) of probable toxic origin. The kidney biopsy, at which time the serum creatinine level was 2.79 mg/dL and for urea 87 mg/dL, showed membranous nephropathy with cellular crescents (see Figure 1A,B).
The patient was treated with boluses of methylprednisolone (500 mg/day) for 3 days. Thereafter, methylprednisolone was changed to prednisone 40 mg/day (1 mg/kg/day). A total of 17 sessions of hemodialysis via jugular vein catheter were needed. Skin lesions and renal function improved significantly 10 days after starting treatment, in conjunction with the withdrawal from cocaine consumption, and there were residual scars. Both blood and urine cultures were negative after treatment with daptomycin for 15 days (4 mg/kg/48 h, in conjunction with the days free of dialysis).
Outcome and follow-up
At the 3-month follow-up (after decreasing doses of prednisone and without toxic consumption of levamisole) skin lesions had subsided and stable analytical parameters were seen both for anemia (Hb: 10.2 g/dL) and renal function (creatinine: 1.98 mg/dL; glomerular filtration rate using the CKD-EPI: 20-30 mL/min; proteinuria <1 g/24 h).
Discussion
Over the last decade, concern about levamisole-adulterated cocaine consumption has increased, given the exponential growth of its illicit use. The reason for its use is justified by its low price as well as the similar physical characteristics to cocaine.
A cocaine/levamisole mixture weighs more while still resembling pure cocaine, therefore increasing its market value. Moreover, levamisole also enhances the biological action of cocaine through aminorex (8). The characteristic cutaneous manifestation after recent cocaine use, i.e., a purple ear lobule with positive perinuclear ANCA serology, allow a differential diagnosis between cutaneous vasculopathy and idiopathic systemic vasculitis in consumers of levamisole-adulterated cocaine (9). Interestingly, these skin lesions were previously described in children treated with levamisole, curiously for the treatment of steroid-resistant nephrotic syndrome (10,11).
The systemic impact of levamisole is of low prevalence but hematological disorders are the most common (12). Furthermore, levamisole is also prothrombotic, lengthening the activated partial thromboplastin time (APTT) by synthesis of antiphospholipid antibodies (13). The second most frequently associated damage from levamisole consumption is pulmonary disease described as pulmonary arterial hypertension (PAH) due to aminorex formation and pulmonary hemorrhage (12). Finally, renal disease incidence is in accordance with some other series of cases (6). From a renal point of view, the clinical diagnosis varies from mild disease with hematuria to renal failure with renal biopsy findings in extracapillary focal pauci-immune glomerulonephritis (14).
In our case, we worked with several possible diagnoses. First, the patient was treated for rapidly progressive glomerulonephritis (RPGN) caused by secondary vasculitis from an infection or toxin (the patient had a history of cocaine consumption and pseudovasculitis), partially excluding primary vasculitis with low complement C3 and C4 levels due to the patient’s rapid renal impairment and sudden onset nephritic syndrome. We rejected the option of a RPGN secondary to endocarditis (necrotic lesions + parenteral drugs user + consumption of complement via the classical pathway) after a TTE without pathologic alterations. The necrotic lesions and the elevation in prothrombotic factors led us to suspect a possible thrombotic microangiopathy. Without knowing the renal function prior to the acute episode, we could not rule out a membranoproliferative glomerulonephritis (MPGN) or GNM secondary to HCV infection, which had worsened after toxin consumption and resulted in acute renal failure. Other possibilities such as a focal segmental glomerulosclerosis secondary to HCV infection, amyloidosis by chronic infection, or even nephrotic syndrome induced by levamisole itself (15) were dismissed due to clinical presentation.
Therefore, renal biopsy findings were quite surprising, becoming the first reported case of GNM and cellular crescents. Although the precise mechanism remains unclear, we determined that the GNM was caused by the deposition of exogenous antigen (levamisole). The cellular crescents probably occurred because of the immunomodulator effect of levamisole and its direct endothelial damage. A limitation of this case is the lack of determination of levamisole levels in a biological sample, but sampling for this could not be performed for ethical reasons. In addition, we also assume that cocaine may also provoke damage through a vasoconstriction mechanism.
After reviewing the literature, it remains contradictory whether the clinical and analytical parameter improvements were a consequence of the corticotherapy at high doses rather than of levamisole consumption cessation. In our opinion, the kidney biopsy could be modified by the early administration of corticosteroids.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mielants H, Veys EM. A study of the hematological side effects of levamisole in rheumatoid arthritis with recommendations. J Rheumatol Suppl 1978;4:77-83. [PubMed]
- Parkinson DR, Cano PO, Jerry LM, et al. Complications of cancer immunotherapy with levamisole. Lancet 1977;1:1129-32. [PubMed]
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc 2012;87:581-6. [PubMed]
- Nakazawa D, Tomaru U, Suzuki A, et al. Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2012;64:3779-87. [PubMed]
- Neynaber S, Mistry-Burchardi N, Rust C, et al. PR3-ANCA-positive necrotizing multi-organ vasculitis following cocaine abuse. Acta Derm Venereol 2008;88:594-6. [PubMed]
- Carlson AQ, Tuot DS, Jen KY, et al. Pauci-immune glomerulonephritis in individuals with disease associated with levamisole-adulterated cocaine: a series of 4 cases. Medicine (Baltimore) 2014;93:290-7. [PubMed]
- Wolford A, McDonald TS, Eng H, et al. Immune-mediated agranulocytosis caused by the cocaine adulterant levamisole: a case for reactive metabolite(s) involvement. Drug Metab Dispos 2012;40:1067-75. [PubMed]
- Szalavitz M. A common cut in cocaine may prove deadly. Time 2010.
- Tran H, Tan D, Marnejon TP. Cutaneous vasculopathy associated with levamisole-adulterated cocaine. Clin Med Res 2013;11:26-30. [PubMed]
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol 1999;140:948-51. [PubMed]
- Powell J, Grech H, Holder J. A boy with cutaneous necrosis occurring during treatment with levamisole. Clin Exp Dermatol 2002;27:32-3. [PubMed]
- Lee KC, Ladizinski B, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol 2012;67:791-2. [PubMed]
- Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol 2011;65:e128-9. [PubMed]
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated disease. Clin J Am Soc Nephrol 2011;6:2799-805. [PubMed]
- Álvarez Díaz H, Marińo Callejo AI, García Rodríguez JF, et al. ANCA-positive vasculitis induced by levamisole-adulterated cocaine and nephrotic syndrome: The kidney as an unusual target. Am J Case Rep 2013;14:557-61. [PubMed]


