Prospective randomized study comparing concomitant chemoradiotherapy using weekly cisplatin & paclitaxel versus weekly cisplatin in locally advanced carcinoma cervix
Introduction
Carcinoma cervix is the most common female malignancy in India with crude incidence rate of 23.5 per 100,000 women per year and of the estimated 134,420 new cases each year; 72,825 women will die partially due to inadequacy of the current treatment (1-3). Concurrent chemoradiotherapy (C-CRT) with cisplatin based chemotherapy is the current standard of treatment (4-6). Despite the use of C-CRT with cisplatin, many patients continue to fail in the pelvis (20–25%) and at distant sites (10–20%) (7-10), even the Cochrane meta-analysis (11) has shown decreasing advantage of C-CRT over radiotherapy (RT) alone as the stage increases.
Striving to improve on these results with Cisplatin based C-CRT, various other single agents and combination chemotherapy has been tried. Theoretically combination chemotherapy with RT could improve local control and survival. The concept has proven helpful in a variety of tumour sites, including the head & neck, lung and others.
Paclitaxel is a taxane alkaloid from pacific yew (Taxus brevifolia) (12) which inhibits tubular aggregation (13,14). Paclitaxel was found to have significant activity in solid tumors especially epithelial ovarian cancer, lung, and breast cancer (15). Preclinical studies have shown a radio-sensitizing effect of paclitaxel in human cervical cancer cell lines (16,17).
The gynaecological oncology group (GOG) reported a 17% response rate using single-agent paclitaxel for advanced squamous cell carcinoma of the cervix (18).
Combination of cisplatin and paclitaxel has been used in metastatic or recurrent carcinoma of cervix in various phase II and III trials with an objective response rate of 36% to 46% (19-21).
In 2011 we began a phase III randomized clinical trial to see the feasibility and benefit with the addition of weekly paclitaxel to the current standard of cisplatin based C-CRT vs. the single agent cisplatin based C-CRT on overall survival (OS) and disease free survival (DFS) at median follow up, local control at 1st follow up and median follow up, and the toxicity profile at 1st follow up in patients with locally advanced carcinoma cervix (stage IIA-IIIB). This study was conceived to act as a validation trial for the use of paclitaxel with cisplatin-chemoradiotherapy for an entirely Asian population of cervical carcinoma.
Methods
Patients
We enrolled women from 18 to 65 years of age, who had stages IIA through IIIB of squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma of the cervix according to the staging system of the International Federation of Gynaecology and Obstetrics (FIGO 2009). Women with a Karnofsky performance score (KPS) of at least 70 and blood counts and serum levels of blood urea nitrogen, creatinine, and bilirubin that were within normal ranges were eligible for the study. Women were excluded from the study if they met any of the following criteria: disease outside the pelvic area or spread to para-aortic lymph nodes; a prior history of malignancy; medical contraindications to chemotherapy; and prior hysterectomy or transperitoneal staging procedure for cervical cancer, pelvic RT, or systemic chemotherapy.
Each patient underwent complete physical examination, including pelvic examination (under anaesthesia if needed) for clinical staging. Other investigations included complete haemogram, blood biochemistry, urine routine & microscopic examination, chest radiography, sonology, & computed tomography (CT) of the abdomen and pelvis. To exclude the bladder and rectal involvement urine cytology, cystoscopy, proctoscopy or intravenous pyelography was done in patients who were either symptomatic or showed bladder or rectum involvement. Patients were required to understand the trial and provide with a written informed consent.
Randomization
The treatment assignment was stratified according to clinical stages of disease. Patients were then randomized by randomization charts, generated from http://www.randomization.com website, into two groups based on treatment they were to receive, one study group where C-CRT was given with weekly cisplatin and paclitaxel (CRT − cis + pacli) and control group where C-CRT was given with weekly cisplatin (CRT − cis). Approximately equal numbers were assigned to each group.
Radiotherapy (RT)
Megavoltage external-beam radiotherapy (EBRT) was administered to a clinical target volume that included the primary cancer, uterus, internal iliac, presacral, upper external iliac, and lower common iliac lymph nodes. This was usually achieved by a “four-field box technique”, or sometimes a parallel-opposed technique. The usual field borders for anterior and posterior fields were superiorly at the L4-L5 inter-space, inferiorly at the bottom of the obturator foramen or 3 cm beyond the disease extent, and laterally 1.5 to 2.0 cm lateral to the bony pelvic wall. Lateral fields had the anterior border at the symphysis pubis and the posterior border at the S2-S3 inter-space, to spare the rectum, however in case of bulky IIIB tumors the border was shifted posteriorly to cover the sacral hollow. No CT simulation was used as it was not available in the department. A dose of 50 Gy was prescribed in 25 equal fractions to the isocenter. Midline shielding with 5 half value layer (HVL) blocks was done after 46 Gy, so that the intracavitary (I/C) dose is not compromised. I/C brachytherapy followed the external-beam RT. Low dose rate (LDR) I/C brachytherapy was given by 137Cs source to dose of 35 Gy to point A in single sitting, taking the total dose to 85 Gy at point A. In case patient was not fit for I/C brachytherapy, she was given supplement EBRT to a dose of 20 Gy/10 fractions (#)/2 weeks with similar portals along with concurrent chemotherapy according to the treatment group.
Concurrent chemotherapy
In the control group cisplatin was given intravenously once a week at a dose of 40 mg/m2 of body-surface area, with the total dose not to exceed 70 mg per week. In the study group paclitaxel was given at a dose of 50 mg/m2 of body surface area along with cisplatin at a dose of 30 mg/m2 of body surface area. Necessary premedication and antiemetics were administered before chemotherapy. Complete haemogram, renal function tests and liver function tests were done weekly before the administration of next cycle of chemotherapy.
Duration
Planned duration of total treatment was 6 to 8 weeks. RT was to be withheld if the patient had a leukocyte count less than 3,000 per mm3 and delays of 1 week were to be allowed in the event of treatment related toxicities. Blood transfusions were given if haemoglobin was <10 g/dL.
Toxicity & follow up
Toxicities were monitored every week and at the end of treatment. Eastern cooperative oncology group (ECOG) toxicity criteria were utilized to assess & document hematologic toxicities & the radiotherapy and oncology group (RTOG) acute morbidity criteria to assess toxicities from RT. First follow up was at 6 weeks after completion of treatment, thereafter 3 monthly for first 2 years, 4 monthly in third year and semi-annually thereafter. Patients were assessed clinically only for response and local failure was documented by performing a biopsy.
Outcome
Statistical analysis including the comparison of survival curves were made by using the log-rank test using SPSS 16.0 while 2×2 tables were assessed using the Fisher exact test for calculation of P values. The primary end points were DFS and OS at median follow up. DFS was calculated from the date of entry into the study to the date of disease recurrence, death, or the last follow-up visit. OS was calculated from the date of entry into the study to the date of death or the last follow-up visit. Recurrences were classified as local if they were detected in the pelvis, cervix, or vagina and as distant if they were detected in extrapelvic locations. Secondary end points were local control, assessed clinically, at first follow up and median follow up; and toxicity, including skin, gastrointestinal (GI), hematological and renal, during treatment and at the end of treatment.
Results
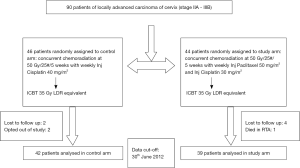
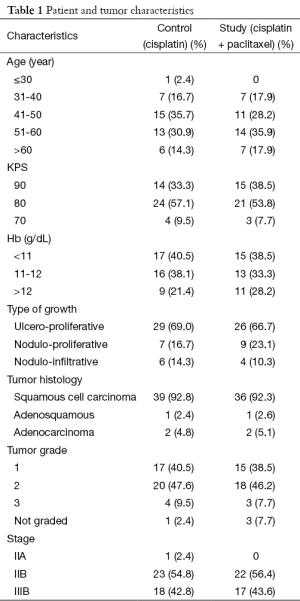
This study was conducted at Regional Cancer Centre, Indira Gandhi Medical College (IGMC), Shimla, Himachal Pradesh, India. Patient enrolment took place from July 2011 to June 2012 for a period of 1 year as a part of limited time protocol. Patient and tumor baseline characteristics are shown in Table 1, no significant differences was seen in these characteristics between the two groups. Out of 90 patients enrolled in this study, 81 patients completed treatment. One patient died in a road traffic accident, six patients were lost to follow up, after completion of external beam radiation, and two patients opted out of study protocol. Of the 81 patients, 42 patients were enrolled in control arm i.e., weekly cisplatin with RT and 39 patients were enrolled in the study arm i.e., weekly cisplatin + paclitaxel with RT, consort diagram is shown in Figure 1.
Full table
Treatment & compliance
RT was delivered according to protocol, with one week treatment break in seven patients due to grade IV toxicity (one hematological and six GI toxicities). Out of these seven patients, five patients were in study group while two patients were in control group. Twelve patients (14.8%) did not undergo brachytherapy, seven in control group and five in study group. The proportion of patients who underwent brachytherapy was similar in the control and the study groups (83.3% vs. 87.2%; P=0.7580). In these patients tandem could not be placed as cervix could not be dilated to accommodate the uterine canal tandem. Median time for completion of radiation was 8 weeks, with a mean dose of 85 Gy being delivered to point A in both the groups. Ten patients did not complete the due five cycles of weekly chemotherapy. The overall compliance for the weekly delivery of concurrent chemotherapy was similar in both the groups, with 90.5% and 84.6% of patients in the control and the study groups respectively receiving the planned schedule of five weekly cycles. Though the difference was not statistically significant (0.5096), it must be acknowledged that the effects of the rather small sample size should not be ignored.
Outcome
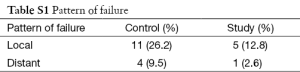
Maximum duration of follow up was 36 months while median duration of follow up was 29 months. Follow up data was available for all the 81 patients studied. Of these, 33 patients in control group (78.6%) and 34 patients in study group (87.2%) were alive at the time of last analysis (Figure 2). Of these 81 patients, 15 patients in control group and eight patients in study group had disease recurrence, thus DFS was 64.3% in control group and 79.5% in study group (Figure 2). Kaplan-Meier analysis revealed that OS rate was not significantly different in these two groups, while DFS had a trend towards improved significance with P value of 0.07. Out of 81 patients, 16 patients had local failure, among them 11 patients were in control group while five patients were in study group with a P value of 0.11. Overall five patients had distant failure with four patients in control group and one patient in study group (Table S1).

Side effects
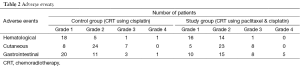
Acute toxicities were monitored for hematological, cutaneous and GI side effects. There were no treatment related deaths however 13 patients (31%) in control group while 22 patients (56.4%) in study group had overall grade III or IV toxicities, a difference which was statistically significant (P=0.026). No difference in grade III and IV toxicities were noticed for hematological and cutaneous toxicities among both the arms (Table 2), however significantly more GI toxicities were seen in the study group leading to more treatment breaks seen in this group.
Full table
Discussion
Carcinoma cervix is the most common female malignancy in developing countries. Due to lack of screening procedures locally advanced carcinoma cervix is a major problem in developing countries, leading to significant morbidity and mortality in female population.
Pelvic RT by itself fails to control the progression of cervical cancer in 35% to 90% of patients with locally advanced disease. Despite improvements in radiation equipment and techniques, in approximately two thirds of the cases, progression occurs within the area that was irradiated (21,22).
Addition of chemotherapy in concurrent setting with radiation has led to moderately improved treatment outcomes (7,8,10,23,24), leading to National Cancer Institute issuing a statement in 1999 stating that “strong consideration should be given to the incorporation of concurrent chemotherapy with radiation for patients who require radiation therapy for the management of cervical cancer” (25).
Despite the use of concurrent chemoradiation with cisplatin it is also recognized that in patients with bulky locoregionally advanced cervical cancer, there remains an appreciable incidence of pelvic relapse, and the risk of distant relapse is as high, if not higher, than pelvic failures following chemoradiation. These findings have led some researchers to propose that adding further chemotherapy to a “backbone” of cisplatin and RT may provide further therapeutic benefit, in terms of both distant and locoregional tumour control. Considering these facts this study was proposed.
Paclitaxel was chosen because preclinical studies have shown a radio-sensitizing effect of paclitaxel in human cervical cancer cell lines (16,17). It was also shown that this drug exerts a preferential cytotoxic activity in human cervical cancer cells with low Raf-1 kinase activity which makes it desirable to be used in conjunction with RT (26). Moreover studies on metastatic and recurrent carcinoma of cervix have also shown a favourable response to paclitaxel (18-21).
Ours was a phase III trial comparing standard of care treatment with cisplatin based chemoradiotherapy to promising combination of cisplatin and paclitaxel based chemoradiation. The randomly assigned treatment arms were well balanced in terms of age, stage, bulk of disease, histology, grade of differentiation, Hb, KPS scores and overall treatment time. A median overall treatment time of eight weeks was achieved which is considered to be adequate by various investigators (22,23).
The inability to deliver I/C brachytherapy in 15% of patients is slightly higher than 10% which is reported in various studies (7,27), and this could be due to use of LDR brachytherapy where tandem is thicker which could not be negotiated through the cervical OS, despite the patient being fit for brachytherapy.
This trial shows that combination of paclitaxel and cisplatin based CRT (study arm) is superior to cisplatin alone based CRT (control arm) in terms of DFS (79.5% vs. 64.3%). There was a trend towards better OS (87.2% vs. 78.6%) in study arm than the control arm, although it was not statistically significant, probably due to relatively small sample size. DFS achieved in this study in the control arm is comparable to other studies using concurrent CRT using cisplatin (28-30), thus signifying for the adequacy of treatment. As delivery of RT was similar in both the arms it is evident that the improvement in DFS should be attributed to addition of paclitaxel based chemotherapy to the standard treatment.
As we had expected before the start of treatment the toxicities in cisplatin and paclitaxel arm were more as compared to the standard cisplatin regimen. GI toxicity mainly appeared in 2nd week of treatment and it was observed that management with frequent hospitalization were more so required in study arm as compared to control arm, although no patient required any surgical intervention and could be managed by conservative methods. Hematological and cutaneous toxicities were comparable. There were more treatment breaks in study arm, but there was no statistical difference between overall treatment time in study arm when compared to control arm and this could be explained due to more patients being fit for timely I/C brachytherapy in study arm, attributable to quicker disease regression.
Pattern of failure
Most of the patients failed locally while few failed in distant sites with para-aortic lymph nodes being the most common distant site of failure. Study group had less number of patients with both local and distant failure (Table S1).
Full table
Shortcomings
The main limitations included the relatively small sample size, which could be attributed to the study being carried out in a single institution which happens to be located in mountain terrain. Due to the study being conducted in a time-bound manner, enrolment was conducted for 1-year only. Further, given the presentation of locally very advanced disease, after completion of the EBRT phase, about 15% of patients did not have adequate regression for them to be qualified for I/C brachytherapy. It can be said at this juncture that similar trials in the future may be conducted with multi-institutional and multi-national collaboration so as to offset the issues concerning limited sample sizes. Also, future trials may benefit from measuring tumor volumetric regression rates to assess quicker disease regression with the experimental regimens. The incorporation of positron-emission tomography based response-evaluation criteria (PERCIST) may improve accuracy of response assessment.
Conclusions
This prospective study demonstrates potential benefit with the addition of paclitaxel to the standard regimen of concurrent cisplatin chemoradiotherapy for carcinoma of the uterine cervix. While an expected increase in toxicities were observed, it must be remarked that the toxicities were manageable. The potential for the improvements in terms of response rates and survival are encouraging, and justify further larger trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre). Human Papillomavirus and Related Cancers in World. Summary Report 2010. [January 15, 2011 accessed]. Available online: http://screening.iarc.fr/doc/Human%20Papillomavirus%20and%20Related%20Cancers.pdf
- Sankaranarayanan R, Nene BM, Dinshaw K, et al. Early detection of cervical cancer with visual inspection methods: a summary of completed and on-going studies in India. Salud Publica Mex 2003;45 Suppl 3:S399-407. [PubMed]
- Shukla S, Bharti AC, Mahata S, et al. Infection of human papillomaviruses in cancers of different human organ sites. Indian J Med Res 2009;130:222-33. [PubMed]
- Thomas GM. Concurrent chemotherapy and radiation for locally advanced cervical cancer: the new standard of care. Semin Radiat Oncol 2000;10:44-50. [PubMed]
- Eifel PJ. Concurrent chemotherapy and radiation therapy as the standard of care for cervical cancer. Nat Clin Pract Oncol 2006;3:248-55. [PubMed]
- Thomas GM. Improved treatment for cervical cancer--concurrent chemotherapy and radiotherapy. N Engl J Med 1999;340:1198-200. [PubMed]
- Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 1999;17:1339-48. [PubMed]
- Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999;340:1154-61. [PubMed]
- Rose PG. Concurrent chemoradiation for locally advanced carcinoma of the cervix: where are we in 2006? Ann Oncol 2006;17 Suppl 10:x224-9. [PubMed]
- Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol 2004;22:872-80. [PubMed]
- Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC). Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database Syst Rev 2010;(1):CD008285. [PubMed]
- Wall ME, Wani MC. Paclitaxel from discovery to clinic. In: George GI, Chen TT, Ojima I, editors. Taxane Anticancer Agent Basic Sciences and Current Status. Washington DC: American Chemical Society, 1995;2:18-30.
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature 1979;277:665-7. [PubMed]
- Pazdur R, Kudelka AP, Kavanagh JJ, et al. The taxoids: paclitaxel (Taxol) and docetaxel (Taxotere). Cancer Treat Rev 1993;19:351-86. [PubMed]
- Curtin JP, Blessing JA, Webster KD, et al. Paclitaxel, an active agent in nonsquamous carcinomas of the uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol 2001;19:1275-8. [PubMed]
- Geara FB, Shamseddine A, Khalil A, et al. A phase II randomized trial comparing radiotherapy with concurrent weekly cisplatin or weekly paclitaxel in patients with advanced cervical cancer. Radiat Oncol 2010;5:84. [PubMed]
- Pradier O, Rave-Fränk M, Schmidberger H, et al. Effects of paclitaxel in combination with radiation on human head and neck cancer cells (ZMK-1), cervical squamous cell carcinoma (CaSki), and breast adenocarcinoma cells (MCF-7). J Cancer Res Clin Oncol 1999;125:20-7. [PubMed]
- McGuire WP, Blessing JA, Moore D, et al. Paclitaxel has moderate activity in squamous cervix cancer. A Gynecologic Oncology Group study. J Clin Oncol 1996;14:792-5. [PubMed]
- Papadimitriou CA, Sarris K, Moulopoulos LA, et al. Phase II trial of paclitaxel and cisplatin in metastatic and recurrent carcinoma of the uterine cervix. J Clin Oncol 1999;17:761-6. [PubMed]
- Rose PG, Blessing JA, Gershenson DM, et al. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol 1999;17:2676-80. [PubMed]
- Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol 2004;22:3113-9. [PubMed]
- Fyles A, Keane TJ, Barton M, et al. The effect of treatment duration in the local control of cervix cancer. Radiother Oncol 1992;25:273-9. [PubMed]
- Lanciano RM, Pajak TF, Martz K, et al. The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: a patterns-of-care study. Int J Radiat Oncol Biol Phys 1993;25:391-7. [PubMed]
- Jampolis S, Andras EJ, Fletcher GH. Analysis of sites and causes of failures of irradiation in invasive squamous cell carcinoma of the intact uterine cervix. Radiology 1975;115:681-5. [PubMed]
- Lanciano RM, Won M, Coia LR, et al. Pretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix: a final report of the 1973 and 1978 patterns of care studies. Int J Radiat Oncol Biol Phys 1991;20:667-76. [PubMed]
- Britten RA, Perdue S, Opoku J, et al. Paclitaxel is preferentially cytotoxic to human cervical tumor cells with low Raf-1 kinase activity: implications for paclitaxel-based chemoradiation regimens. Radiother Oncol 1998;48:329-34. [PubMed]
- Thomas G, Dembo A, Ackerman I, et al. A randomized trial of standard versus partially hyperfractionated radiation with or without concurrent 5-fluorouracil in locally advanced cervical cancer. Gynecol Oncol 1998;69:137-45. [PubMed]
- Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999;340:1144-53. [PubMed]
- Pearcey RG, Brundage MD, Drouin P, et al. A clinical trial comparing concurrent cisplatin and radiation therapy versus radiation alone for locally advanced squamous cell carcinoma of the cervix carried out by the National Cancer Institute of Canada Clinical Trials Group. Proc Am Soc Clin Oncol 2000;19:378a.
- Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 1999;340:1137-43. [PubMed]