Diagnosis value of CD117 and PDGFRA, alone or in combination DOG1, as biomarkers for gastrointestinal stromal tumors
Introduction
The gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the intestinal tract, accounting for about 70% of all gastrointestinal mesenchymal tumors (1-3). GIST occurs mainly in middle-aged and elderly people and can affect both men and women (4). In past, the understanding of GIST diagnosis is quite diverse due to limitations in pathological techniques. Research has demonstrated that most of the previously diagnosed gastrointestinal smooth muscle tumor and neurilemmoma belong to GIST (5,6). In recent years, GIST has become a hot research topic along with advances in immunohistochemical and molecular pathological studies and with the development of the molecularly targeted drug imatinib (Gleevec). The discovered on GIST-1 (DOG1) is a specifically expressed membrane protein in GIST cells. It is encoded by the DOG1 gene, while its specific functions remain unclear (7,8). In our current study, we retrospectively analyzed the clinicopathological parameters in 99 GIST patients, with an attempt to explore the role of CD117 and PDGFRA proteins, alone or in combination with DOG1 protein, in diagnosing GIST.
Patients and methods
Patients
Totally 99 patients with surgically/pathologically confirmed GIST who were surgically treated in our centers from 2012 to 2013 were enrolled as the GIST group; in addition, 25 patients without GIST were enrolled as the non-GIST group. In the GIST group, there were 51 men and 48 women, with a median age at onset of 56 years (range, 26-85 years). The tumors sized 5-45 cm (median: 6.2 cm). The primary tumor sites included stomach (n=50), small intestine (n=28), and colon and rectum (n=21). The histological types included spindle cell type (n=74), epithelial cell type (n=13), and mixed type (n=12). The risk degree was extremely low in 12 patients, low in 29 patients, moderate in 37 patients, and high in 21 patients [according to the National Institute of Health risk stratification method (9)]. In the non-GIST group, there were 13 men and 12 women, with a median age at onset of 49 years (range, 14-72 years). The histological types included smooth muscle tumors (n=17) and neurilemmoma (n=8). The primary tumor sites included stomach (n=9), small intestine (n=8), and colon and rectum (n=8). Each specimen from these patients was reviewed by three pathologists.
Methods
The postoperative specimens were fixed in 4% neutral-buffered formalin prior to routine dehydration and embedment. Paraffin sections were cut at 3 µm thickness for routine HE staining. The expressions of relevant proteins were detected by immunohistochemistry (EnVision method). The anti-CD117, anti-PDGFRA, and anti-DOG1 antibodies and EnVision kit were purchased from ZSQB-Bio, Beijing, China. The experimental procedures were conducted in strict accordance with kit instructions. Positive controls were set for each batch of stained specimens, and PBS was used as the negative control.
Result judgment
CD117 is the protein product of carcinoma-in-situ gene, with the positive sites being the cytoplasm and nucleus. PDGFRA is the protein product of carcinoma-in-situ gene, with the positive sites being the cell membrane and cytoplasm. DOG1 can be selectively expressed in GIST, with the positive sites being the cell membrane and cytoplasm. The criteria for positive staining (brown-yellow in color) is as follows (10): randomly select 10 high-power fields and count the number of positive cells, and then calculate the average. According to the proportion of positive cells among the tumor cells, the results were interpreted as follows: −, if <5%; +, if 5-25%; ++, if 26-50%; and +++, if >50%.
Statistical analysis
The statistical analysis was performed using the SPSS 19.0 software package. Using chi square test and Fisher’s exact probability method, we calculated the sensitivities, specificities, and areas under ROC curve (AUC) of CD117, PDGFRA, DOG1, CD117 + DOG1, PDGFRA + DOG1, and CD117 + PDGFRA + DOG1 in detecting/judging GIST, with a statistical significance level of α=0.05. A two-sided P<0.05 was considered statistically significant.
Results
Protein expressions of CD117, PDGFRA, and DOG1 in gastrointestinal stromal tumor (GIST) group
The positive rates of CD117, PDGFRA, and DOG1 in GIST group [93.94% (93/99), 53.54% (53/99), and 90.91% (90/99), respectively] were significantly higher than those in non-GIST group [4.00% (1/25), 4.00% (1/25), 12.00% (3/25), respectively] (χ2=83.198, P=0.000; χ2=17.761, P=0.000; and χ2=62.142, P=0.000, respectively) (Figure 1).
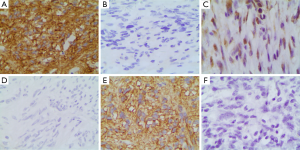
Relationship between the protein expressions of CD117, PDGFRA, and DOG1 and clinicopathological parameters in gastrointestinal stromal tumor (GIST) group
The expressions of CD117, PDGFRA, and DOG1 had no significant correlation with clinicopathological parameters including gender, age, tumor diameter, tumor location, histotype, and risk degree (all P>0.05) in GIST patients (Table 1).
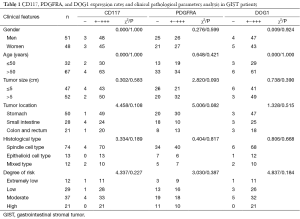
Full table
Sensitivities and specificities of CD117 and PDGFRA, alone or in combination with DOG1, in detecting gastrointestinal stromal tumor (GIST)
The sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, odds ratio, and AUC of CD117 and PDGFRA, alone or in combination with DOG1, in detecting GIST are listed in Table 2.
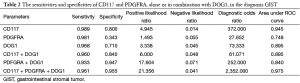
Full table
Role of ROC curve in gastrointestinal stromal tumor (GIST) diagnosis
The AUCs of CD117, PDGFRA, DOG1, CD117 + DOG1, PDGFRA + DOG1, and CD117 + PDGFRA + DOG1 in detecting/judging GIST were 0.945, 0.748, 0.895, 0.895, 0.840, and 0.975. It was significantly in the CD117+ PDGFRA + DOG1 group than those in the other five groups (Figure 2).
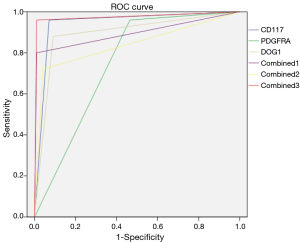
Discussion
GISTs are rare neoplasms arising from gastrointestinal mesenchymal tissues, with a prevalence of about 0.5-1 per 100,000 persons in the general population (1). The diagnosis of GIST is mainly based on the pathological and immunohistochemical findings. Pathologically the GIST cells have two morphological types: spindle cells and epithelioid cell types. According to the histological features, GIST can be divided into three types: spindle cell type, epithelioid cell type, and mixed type. Under the microscope, the spindle cells are often found to be arranged in a form of cross-beam, fence, or whorl, with vacuoles commonly seen around the nucleus, which makes it difficult to differentiate it from smooth muscle-originated tumors, fibrous tumors, and neurogenic tumors. The epithelioid cells are often ranged in diffuse, nesting forms. Their cytoplasm is deeply eosinophilic, with vacuoles with translucent content. The features of nucleus can be diverse. Sometimes it is difficult to differentiate it from atypical leiomyoma and Signet Ring cells. The mixed type is defined by the co-existence of spindle cells and epithelioid cells. The spindle cell type is most common, accounting for about 70%; the epithelioid cell type is less frequently seen, accounting for 20%; even less is the mixed type (11). Immunohistochemically, CD117 is the protein product of c-Kit gene, with a protein expression rate of 80-100% in GIST but seldom expressed in non-GIST (12). PDGFRA is the product of PDGFRA gene activation and mutation, with a protein expression rate of 50-60% in GIST; compared with CD117, it has relatively lower sensitivity and specificity (13). It has been reported that 4-15% of GIST patients had negative or unconfirmed CD117 or PDGFRA expression, which may mislead the diagnosis of GIST (14). DOG1 is a novel biomarker that is specifically expressed in GIST; also, it is a transmembrane protein on human 11q13 chromosome. It has a high protein expression rate (94-96%) in GIST cells but usually is not expressed in other tissues (7,15,16). In our current study, the protein expressions of CD117, PDGFRA, and DOG1 were significantly higher in GIST group than in non-GIST group. However, the expressions of CD117, PDGFRA, and DOG1 of GIST patients had no significant correlation with clinicopathological parameters including gender, age, tumor diameter, tumor location, histotype, and risk degree (all P>0.05). Thus, the populations that may benefit more from the detection of CD117, PDGFRA, and DOG1 protein expression for GIST need to be further identified.
Dei Tos et al. (17) detected DOG1 expression in specimens obtained from 139 GIST patients and 438 non-GIST patients and found that the sensitivity of DOG1 expression in judging GIST was up to 97.84%; meanwhile, they also found positive DOG1 expression in CD117-negative patients, suggesting that combination of DOG1 and CD117 may be more valuable for GIST detection. To our knowledge, no study had explored the roles of PDGFRA + DOG1 and CD117 + PDGFRA + DOG1 in detecting GIST. In our current study, the sensitivity, specificity, diagnostic OR, and AUC of CD117 protein expression in detecting GIST were 0.989, 0.800, 372.000, and 0.945, respectively; the sensitivity, specificity, diagnostic OR, and AUC of PDGFRA protein expression in detecting GIST were 0.981, 0.343, 27.652, and 0.748, respectively; the sensitivity, specificity, diagnostic OR, and AUC of DOG1 protein expression in detecting GIST were 0.968, 0.710, 73.333, and 0.895, respectively; the sensitivity, specificity, diagnostic OR, and AUC of CD117 + DOG1 protein expressions in detecting GIST were 0.960, 0.840, 61.071, and 0.895, respectively; the sensitivity, specificity, diagnostic OR, and AUC of PDGFRA + DOG1 protein expressions in detecting GIST were 0.933, 0.947, 252.000, and 0.840, respectively; and the sensitivity, specificity, diagnostic OR, and AUC of CD117 + PDGFRA + DOG1 protein expressions in detecting GIST were 0.961, 0.955, 352.000, and 0.975, respectively. Therefore, detection of CD117 and PDGFRA protein, alone or in combination with DOG1, may increase the accuracy of GIST diagnosis.
In summary, the populations that may benefit more from the detection of CD117, PDGFRA, and DOG1 protein expression for GIST need to be further identified. Detection of CD117 and PDGFRA protein, alone or in combination with DOG1, may increase the accuracy of GIST diagnosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Reichardt P, Morosi C, Wardelmann E, et al. Gastrointestinal stromal tumors: evolving role of the multidisciplinary team approach in management. Expert Rev Anticancer Ther 2012;12:1053-68. [PubMed]
- Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet 2007;369:1731-41. [PubMed]
- Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res 2001;61:8118-21. [PubMed]
- Blay JY, Le Cesne A, Cassier PA, et al. Gastrointestinal stromal tumors (GIST): a rare entity, a tumor model for personalized therapy, and yet ten different molecular subtypes. Discov Med 2012;13:357-67. [PubMed]
- Miettinen M, Lasota J, Sobin LH. Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol 2005;29:1373-81. [PubMed]
- Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc 2009;69:1218-23. [PubMed]
- Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol 2009;33:1401-8. [PubMed]
- Hwang DG, Qian X, Hornick JL. DOG1 antibody is a highly sensitive and specific marker for gastrointestinal stromal tumors in cytology cell blocks. Am J Clin Pathol 2011;135:448-53. [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [PubMed]
- Faigel DO, Abulhawa S. Gastrointestinal stromal tumors: the role of the gastroenterologist in diagnosis and risk stratification. J Clin Gastroenterol 2012;46:629-36. [PubMed]
- Min KW. Small intestinal stromal tumors with skeinoid fibers. Clinicopathological, immunohistochemical, and ultrastructural investigations. Am J Surg Pathol 1992;16:145-55. [PubMed]
- Xiaohong Liu, Dalie Ma, Lili Wu, et al. Expression and significance of c-Kit oncogene in gastointestinal stremal tumors. Chinese Journal of Surgery 2002;40:277-9.
- Qinling Sui, Wang Hao, Xiuwei Sun. DOG1, CD117 and PDGFRA expression and correlation in gastrointestinal stromal tumor. World Chinese Journal of Digestology 2011;19:912-8.
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708-10. [PubMed]
- Novelli M, Rossi S, Rodriguez-Justo M, et al. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology 2010;57:259-70. [PubMed]
- Liegl B, Hornick JL, Corless CL, et al. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol 2009;33:437-46. [PubMed]
- Dei Tos AP. The reappraisal of gastrointestinal stromal tumors: from Stout to the KIT revolution. Virchows Arch 2003;442:421-8. [PubMed]


