Early surgical and functional outcomes comparison of the supercapsular percutaneously-assisted total hip and traditional posterior surgical techniques for total hip arthroplasty: protocol for a randomized, controlled study
Introduction
Total hip arthroplasty (THA) has been performed since the early 1920s and is generally considered to be one of the most successful orthopaedic surgeries. The 11th Annual Report from the National Joint Registry of England and Wales reported 96.2% component survivorship at 10 years for over 600,000 THAs (1). While the long-term success of THA is well documented, there is still significant room for improving patient speed of recovery and return to activities. The surgical technique used during THA has the potential to affect these early outcomes.
The supercapsular percutaneously-assisted total hip (SuperPath®) surgical technique (MicroPort Orthopedics Inc., Arlington, TN, USA) is a tissue-sparing approach that combines aspects of the SuperCap® and PATH® surgical technqiues (MicroPort Orthopedics Inc., Arlington, TN, USA) (2). This technique allows access to the hip capsule without requiring the cutting of tendons or muscles. The lack of collateral soft tissue damage has been shown to contribute to shorter length of stay (LOS), more patients being discharged directly home, and reduced 30-day readmission rates when compared to averages reported in the United States (3).
While these early results compared to published averages in the United States are promising, further studies are needed to compare results for this technique to traditional techniques in a controlled study. The purpose of the current study is to compare the perioperative outcomes, pain relief, and return to function of the SuperPath technique with the traditional posterior surgical technique. The traditional posterior technique was selected as a comparator, as it is currently the most commonly used approach for THA.
Methods
Study design and study groups
The selected study design is a single center, two-surgeon, randomized, controlled study of primary THA subjects implanted using two different surgical techniques. There will be two study groups based upon the surgical technique used to perform the THA. Group 1 will consist of subjects implanted using the SuperPath surgical technique by one surgeon proficient with the technique. Group 2 will consist of subjects implanted using the posterior surgical technique by a second surgeon proficient with that technique. Subjects in both groups will be implanted with the same THA implants (i.e., acetabular component, acetabular liner, femoral component, femoral head).
Study objectives
The primary objective of the study is to compare the Timed Up and Go (TUG) times for the two study groups at discharge from the hospital. Secondary objectives include:
- To assess and compare how subjects in each group return to function, as indicated by TUG, Timed Stair Climb (TSC), and the Hip Dysfunction and Osteoarthritis Outcome Score (HOOS) questionnaires at each follow-up interval.
- To assess and compare pain levels and relief during the hospital stay and for the first 100 days following THA.
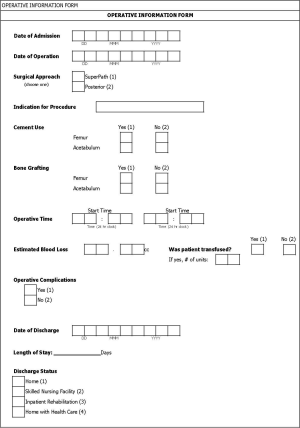
- To assess and compare operative outcomes for the two groups including: LOS; acetabular component inclination and anteversion angles; skin-to-skin operative times; date returned to work; transfusion rates; and discharge status.
- To assess and compare adverse events, complication rates, and 30-, 60-, and 90-day readmission rates for the two groups.
Follow-up intervals and outcomes measures
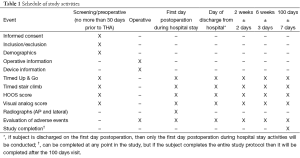
The schedule of study activities and their associated follow-up intervals are shown in Table 1. Subjects will be seen preoperatively no more than 30 days prior to the THA procedure, during the hospital stay, and during three follow-up visits following hospital discharge. Evaluations performed during the subject’s hospital stay will occur on the first day following the THA procedure and again on the day of discharge. If a subject is discharged on the first day after the THA procedure, then they will only be evaluated once during the hospital stay. Post-discharge visits following THA will occur at 2 weeks ± 2 days, 6 weeks ± 3 days, and 100±7 days.
Full table
Sample size
The primary objective of this study is to compare TUG times during the hospital stay for two surgical techniques. A previous study comparing TUG times for a minimally-invasive surgical technique and the posterior technique reported the mean TUG time during the hospital stay was 34±17 s for the minimally-invasive group and 60±36 s for the posterior group (4). Using these values as surrogates for the two techniques in the current study, the sample size was calculated as 25 subjects in each group. This sample size will allow for the detection of a difference in means of 26 s, assuming power of 80% and one-sided significance of 0.05.
Inclusion criteria
To be included in the study, subjects must meet all of the following criteria:
- Subject is a candidate for primary THA for any of the following:
- Non-inflammatory degenerative joint disease including osteoarthritis, traumatic arthritis, or avascular necrosis;
- Inflammatory degenerative joint disease including rheumatoid arthritis;
- Correction of functional deformity.
- Subject is skeletally mature (21 years of age or older);
- Subject is a candidate to be implanted with the specified combination of components;
- Subject is willing and able to complete required study visits and assessments;
- Subject plans to be available through the 100 day follow-up visit;
- Subject is willing to sign the approved informed consent document.
Subjects with a previous THA in the contralateral hip are eligible for enrollment provided (I) it has been at least 1 year since the contralateral THA and (II) the contralateral THA is asymptomatic and not pending revision. Simultaneous bilateral THA subjects will not be permitted to enroll.
Exclusion criteria
Subjects will be excluded if they meet any of the following criteria:
- Subject has overt infection or distant foci of infections;
- Subject has rapid disease progression as manifested by joint destruction or bone absorption apparent on roentgenogram;
- Subject with inadequate neuromuscular status (e.g., prior paralysis, fusion and/or inadequate abductor strength), poor bone stock, poor skin coverage around the joint which would make the procedure unjustifiable;
- Subject has a neuropathic joints;
- Subject has hepatitis or HIV infection;
- Subject has a neurological or musculoskeletal disease that may adversely affect gait or weight-bearing;
- Subject is currently enrolled in another clinical investigation that could affect the endpoints of this study;
- Subject is unwilling or unable to sign the informed consent document;
- Subject has documented substance abuse issues;
- Subject has a body mass index (BMI) of greater than 40;
- Subject is currently incarcerated or has impending incarceration.
Enrollment procedures
Investigators, or designees, will compile a list of potential subjects by reviewing records of patients in their care currently awaiting THA. Once a potential subject is identified, the Investigators, or designees, will inform them of all aspects of the study, including potential risks and benefits. Potential subjects will be allowed ample time to consider and ask questions about the information they have been provided. If after being informed the potential subject still wishes to participate, they will be asked to sign an informed consent document. Once the subject has signed the informed consent document and has met the inclusion/exclusion criteria they will be considered enrolled in the study. Any subject who signs the informed consent document and fails to satisfy the inclusion/exclusion criteria will be considered a screen failure. Enrolled subjects will be randomized using 1:1 assignment determined by a random number generator. Investigators, or designees, will continue to randomize subjects until they have fulfilled the enrollment of 25 subjects in each group. Blinding of subjects and research staff is not feasible for the current study, as the surgeon will have to know which procedure to perform and the subject will know the technique used based upon the size and location of the incision.
Study outcome measures
Example case report forms can be found in the supplementary materials for all measures except the HOOS Scores which are available on www.koos.nu. Study outcome measures will include the following:
- TUG: the TUG is a timed physical examination that evaluates the time it takes a subject to stand from a seated position, walk 3 meters, and return to a seated position.
- TSC: the TSC is the time needed to go up and down a flight of 12 stairs.
- HOOS: the HOOS is a patient reported outcome measure developed to evaluate a subject’s opinion about the status of their hip. It consists of 40 questions grouped in 5 subcategories: symptoms, pain, daily living, sports and recreational activities, and quality of life. The HOOS was selected for use in this study because it has been validated for THA, evaluates several aspects of hip function, and does not require a license for use making it easier for other researchers to replicate the study (5,6).
- 10-point VAS: a 10-point scale for measuring a subject’s pain level. Subjects will be asked to rate their pain between 0 meaning “No Pain” and 10 meaning “Worst Pain Imaginable”.
- Radiographs: standard anterior-posterior (AP) and lateral radiographs will be collected to measure acetabular component inclination and anteversion angles. This was selected as an outcome measure because certain surgical techniques have been shown to be associated with poor alignment of acetabular components (7).
- Operative time: operative time is defined as the time between the first skin incision and the completion of closing the skin following the surgery.
- Transfusion rate: transfusion rate is the percentage of subjects requiring a blood transfusion during or after the procedure prior to discharge from the hospital.
- Discharge status: discharge status indicates where each subject is discharged and can include the following options: home; skilled nursing facility; inpatient rehabilitation; or home with health care.
- LOS: LOS is defined as the number of nights the subjects stays in the hospital. If the subject is admitted and discharged on the same day, then LOS is zero days.
- The 30-, 60-, and 90-day readmission rates: the percentage of subjects readmitted to the hospital for any reason following discharge at 30-, 60-, and 90-day.
Preoperative study procedures
Following the consent process, the investigator or designee will perform the following measurements: TUG, TSC, HOOS, and VAS. These measurements will occur no more than 30 days prior to the subject’s THA procedure. Relevant subject medical history and demographics will be collected during this visit. This includes date of birth, gender, height, weight, status of other joints that affect ambulation, previous treatment to the enrolled hip, and concomitant medical issues.
Operative and in-hospital study procedures
Subjects in group 1 will be implanted using the SuperPath surgical technique, while those in group 2 will be implanted using the posterior surgical technique. The date of operation, primary diagnosis for the THA procedure, skin-to-skin operative time, and intraoperative complications will be collected related to the THA operation. The TUG, TSC, HOOS, VAS, standard AP and lateral radiographs, transfusions, adverse events, and discharge status will be collected during the in-hospital stay in accordance with Table 1.
Post-discharge study procedures
At each of the three follow-up intervals, the TUG, TSC, HOOS, VAS, and any adverse events will be collected. Additionally, the Investigators or designee will report if subjects have been readmitted to the hospital for any reason during the study duration.
Study ethics
Prospective approval of the clinical protocol, any clinical protocol amendments, informed consent document, and any other relevant documents will be obtained from the Ethics Committee (EC) prior to the screening of potential subjects.
Protocol amendments
Amendments to the protocol must be approved by the EC prior to their implementation.
Protocol deviations
Investigators will not deviate from the clinical protocol except to deliver emergency care or to eliminate an immediate hazard to the subject. All deviations with the potential to affect subject safety, rights, or well-being must also be reported as required by the EC.
Subject enrollment completion
Individual subject participation will conclude once the subject has completed all required clinical visits and all outcome measurements required by the clinical protocol. The date of last follow-up, the date of study withdrawal, or date subject was determined to be lost to follow-up will be recorded.
Study closeout activities
The study will be considered complete once the last subject has completed all visits and all outcome measurements required by the clinical protocol. Additionally, the following activities must be completed before the study is considered complete:
- All essential documents are complete and up to date;
- Current status or resolution of all ongoing serious adverse events and adverse device effects is documented;
- Arrangements are made for archiving and record retention according to local and any regulatory requirements;
- The EC has been notified of the conclusion of the study;
- All adverse events have been recorded and the outcome verified and/or updated, as applicable.
Study duration
The anticipated duration of the study, including enrollment time, is 2 years.
Statistical analysis plan
Demographics and operative outcomes will be summarized using descriptive statistics. Continuous variables (e.g., TUG, TSC, HOOS, and VAS) will be summarized using mean, standard deviation, minimum, maximum, and median values. Frequency counts and percentages of subjects within each category will be provided for categorical data. Outcomes for the two groups will be compared using analysis of variance (ANOVA). A paired t-test will be used to compare the change from baseline for outcomes in each group. All analyses will be performed using SAS Software Version 9.1 or later (SAS Institute Inc., Cary, NC, USA).
Discussion
Previously published early results are promising for the SuperPath surgical technique (2,3,8), but to date there have been no studies comparing to other techniques in a controlled patient population. The described study protocol will be the first to do so. Ideally, there would be a single surgeon randomizing subjects to receive THA using the two surgical techniques to minimize as many variables as possible. However, outcomes of THA can be heavily influenced by surgeon proficiency with a given technique. The authors felt the best way to minimize this impact was to have surgeons who perform these techniques regularly in the majority of their patients perform them as part of the study. Another potential limitation is the single center design. The single center design was selected because some endpoints of the study (e.g., LOS, discharge status, transfusion rates) can be highly dependent upon the staff and protocols in place at individual hospitals. A single center design allows for subjects to be treated according to identical protocols and have that care provided by the same staff. In conclusion, the described study will evaluate outcomes of a tissue-sparing surgical technique for THA in comparison to the technique most commonly used in clinical practice while removing several potential sources of bias.
Supplementary
Acknowledgements
None.
Footnote
Conflicts of Interest: L Erwin and DA Fitch are paid employees of MicroPort Orthopedics Inc.; W Gofton and J Chow receive payment from Microport Orthopedics on a fee-for service basis for education; MD Cronin has no conflicts of interest to declare.
References
- 11th Annual Report of the National Joint Registry of England, Wales, and Northern Ireland. 2014. Available online: http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/11th_annual_report/NJR%2011th%20Annual%20Report%202014.pdf
- Chow J, Penenberg B, Murphy S. Modified micro-superior percutaneously-assisted total hip: early experiences & case reports. Curr Rev Musculoskelet Med 2011;4:146-50. [PubMed]
- Gofton W, Chow J, Olsen KD, et al. Thirty-day readmission rate and discharge status following total hip arthroplasty using the supercapsular percutaneously-assisted total hip surgical technique. Int Orthop 2015;39:847-51. [PubMed]
- Rodriguez JA, Deshmukh AJ, Rathod PA, et al. Does the direct anterior approach in THA offer faster rehabilitation and comparable safety to the posterior approach? Clin Orthop Relat Res 2014;472:455-63. [PubMed]
- Nilsdotter AK, Lohmander LS, Klässbo M, et al. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total hip replacement. BMC Musculoskelet Disord 2003;4:10. [PubMed]
- Klässbo M, Larsson E, Mannevik E. Hip disability and osteoarthritis outcome score. An extension of the Western Ontario and McMaster Universities Osteoarthritis Index. Scand J Rheumatol 2003;32:46-51. [PubMed]
- Callanan MC, Jarrett B, Bragdon CR, et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop Relat Res 2011;469:319-29. [PubMed]
- Gofton W, Fitch DA. In-hospital cost comparison between the standard lateral and supercapsular percutaneously-assisted total hip surgical techniques for total hip replacement. Int Orthop 2015. [Epub ahead of print]. [PubMed]