
Vitamin D deficiency inhibits microRNA-196b-5p which regulates ovarian granulosa cell hormone synthesis, proliferation, and apoptosis by targeting RDX and LRRC17
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disease in premenopausal women, with a prevalence rate of about 6–7%. PCOS is characterized by clinical or biochemical hyperandrogenemia, polycystic ovary morphology, chronic anovulation, or less menstruation (1,2). PCOS is considered hereditary and is caused by a variety of factors, such as increased luteinizing hormone (LH), lack of androgen balance, inflammation, insulin resistance (IR), and oxidative stress (OS) (3). Vitamin D deficiency (VDD) is common in women with PCOS. It is estimated that serum 25-hydroxyvitamin D [25(OH)D] is less than 20 ng/mL in 65–85% of patients with PCOS (4). Notably, VDD may aggravate the symptoms of PCOS because lower concentrations of 25(OH)D are associated with irregular ovulation and menstruation, hyperandrogenemia, IR, hirsutism, low success rate of pregnancy, obesity, and increased risk factors for cardiovascular disease (5,6). There are several methods for the fertility treatment of patients with PCOS. Assisted reproductive technology (ART) is the most commonly used method. In this method, the ovaries are stimulated by exogenous gonadotropin to produce multiple follicles. A large amount of evidence suggests that vitamin D (VD) may have beneficial effects on metabolic/hormonal parameters in PCOS and endometriosis, and seems to be related to the results of in vitro fertilization (IVF) (7-9). In PCOS, ovarian physiology is tightly linked to the metabolic disturbances observed in this disorder. Thus, the beneficial effects of VD on metabolic alterations may translate into better and healthier ovarian physiology.
Radixin (RDX) is a member of the Ezrin-Radixin-moesin (ERM) protein family. It plays the role of membrane cytoskeleton crosslinker in actin rich cell surface structures. Therefore, it is considered to be essential for the organization, cell movement, adhesion, and proliferation of the cortical cytoskeleton. They are also involved in regulating the transporter function of various organs. For example, RDX knockout mice showed binding hyperbilirubinemia and liver damage caused by loss of the bilirubin-secreting transporter multidrug resistance-associated protein 2 (MRP2) in the bile duct membrane (10). leucine rich repeat containing 17 (LRRC17) is a secretory protein containing 5 leucine-rich repeat domains. It was first characterized in bone metabolism as an inhibitor of receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclast differentiation. Besides bone metabolism, Malcuit et al. found that LRRC17 was particularly high expressed in oocytes and granulosa cells (GCs) (11). Although human protein maps showed that LRRC17 is highly expressed in female tissues, but its role in GCs remains unclear.
MicroRNAs (miRNAs) are a class of small non-coding RNAs. It is estimated that they regulate the mRNA translation of more than 70% of protein-coding genes and are widely involved in normal and disease states (12). In addition, miRNAs are involved in the regulation of GCs proliferation, apoptosis, and steroid production, and their imbalance may play an important role in the pathogenesis of PCOS (13,14). Some studies have determined that VD3 affects the expression of genes involved in steroid production in normal ovarian GCs (15-17). However, there are no studies showing the effects of miRNAs on GC proliferation, apoptosis, OS, glucose uptake, or the steroid production pathway under VDD conditions. In this study, for the first time, we analyzed the transcriptome of the ovaries in a VDD mouse model and evaluated the effect of miR-196b-5p on the hormone synthesis, proliferation, and apoptosis of GCs by targeting RDX and LRRC17. We present the following article in accordance with the ARRIVE reporting checklist (available at https://dx.doi.org/10.21037/atm-21-6081).
Methods
Animal procedure
Female C57BL/6J mice (age, 3 weeks; weight, 12–18 g) were obtained from Carvens, Inc. (Changzhou, China) [animal certificate No. SCXK(SU)2016-0010]. Mice were housed in a specific pathogen-free environment under standard housing conditions with controlled temperature (22±1 °C) and humidity (52%±5%). The mice were exposed to a 12 h light/dark cycle and were able to access a specified amount of food and water at will. All animal experiments in this study were carried out in strict accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People’s Republic of China (11-14-1988). The animal experimental design and protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University (approval number: 2020-62).
The experimental group and procedures of mice were described previously (18). In the present study, a total of 30 mice were used, of which 5 were the control group and 5 were VD deficient group, and repeat for 3 times independently to eliminate the difference between experiments at different batches. Briefly, after feeding for 8 weeks with a VD-deficient (25 IU VD3/kg; cat. no. D119289, Dyets) or control diet (5,000 IU VD3/kg; cat. no. D119290, Dyets), mice were sacrificed by cervical dislocation. Before analysis, the serum samples were centrifuged at 6,000 rpm for 5 minutes and stored at −20 °C. Serum 25(OH)D3 levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (cat. no. AC-57SF1; Immunodiagnostic Systems, Ltd.). Determination of serum levels of follicle stimulating hormone (FSH), LH, estradiol, and testosterone was performed using commercial ELISA kits (Tongwei Bio, Shanghai, China). Ovarian tissues were collected in 4% formalin for hematoxylin and eosin (H&E) staining or RNAlater (Invitrogen, USA) for RNA sequencing by Genewiz, Inc. (Suzhou, China), as described previously (19).
Cell culture
KNG cells were obtained from Nanjing Medical University and cultured in Dulbecco’s Modified Eagle’s Medium/F12 (DMEM/F12; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA) in an incubator with 5% CO2 at 37 °C. At the time of passage, the cells were first digested with 0.25% trypsin (containing EDTA). Subsequently, the cell suspension was evenly distributed into a cell culture flask, with the whole medium supplemented to 6 mL per flask. Cell culture was then continued for subsequent experiments.
Cell counting kit-8 (CCK-8) assay
KGN cells were cultured at a density of 0.3-1×105 cells/well in 24-well culture plates and transfected with 50 nM miR-196b-5p mimics/ mimics control and miR-196b-5p inhibitor/ inhibitor control using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to instruction of the manufacturer. After transfection, cells in log phase were trypsinized and resuspended in complete medium, and 100 μL of cell suspension was added to the culture plate at a density of 5×103 cells/per well. After culture for 0, 24, 48, and 72 h, 100 μL of DMEM solution containing 10% CCK-8 reagent (Solarbio, Beijing, China) was added to each well, followed by incubation in an incubator for 2 h in the dark. Lastly, absorbance at 450 nm was detected using a preheated microplate reader.
Flow cytometry
Cells were first cultured in 0.5% Roswell Park Memorial Institute-1640 (RPMI-1640) medium (HyClone, South Logan, UT, USA) for 48 h. Then, the medium was replaced with 10% RPMI-1640 medium for another 48 h. Subsequently, the cells were collected in 3 mL tubes with approximately 2×106 cells per tube. After washing twice with phosphate-buffered saline (PBS) solution, the cells were fixed with 70% alcohol and placed in a refrigerator at −20 °C for 24–48 h. After routine PI staining, DNA content was measured by AttuneNxT flow cytometry (Thermo, USA). Finally, the percentage of each stage of the cell cycle was analyzed by CytExpert software.
Reverse transcription‑quantitative PCR (RT-qPCR)
Total RNA was extracted from KGN cells by Trizol reagent (Invitrogen, Carlsbad, CA, USA). Using 100 ng of total RNA as a template, cDNA was obtained through the TaqMan microRNA reverse transcription kit (Applied Biosystems, Carlsbad, CA, USA). According to the manufacturer’s instructions, the qPCR reaction was performed using the ABI7900 real-time PCR system (ABI, Carlsbad, CA, USA). PCR primers (Table 1) were designed and synthetized by Sangon Biotech (Shanghai, China). Actin served as the internal reference (U6 served as the internal reference for endonuclear RNA and miR-196b-5p). The threshold was selected at the lowest point of each logarithmic magnification curve to obtain the threshold cycle (Ct) value of each reaction tube, and the data were analyzed by the 2−ΔΔCt method.
Table 1
| Gene | Sequences (5'-3') |
|---|---|
| U6 R | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATA |
| U6 F | AGAGAAGATTAGCATGGCCCCTG |
| MiR-196b-5p R | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCCAACAA |
| MiR-196b-5p F | ACACTCCAGCTGGGTAGGTAGTTTCCTGTT |
| Actin F | TGGACTTCGAGCAAGAGATG |
| Actin R | GAAGGAAGGCTGGAAGAGTG |
| RDX F | CACCTCCACCACCACCAGTC |
| RDX R | TTCAGCATTATTCTCATCGTGTTCATC |
| LRRC17 F | TAAACAAACTCACCACCCTCTTACTG |
| LRRC17 R | TGGACTTTCACACTTGGCGTAG |
| CYP19A1 F | GACACCTCTAACACGCTCTTCTTG |
| CYP19A1 R | AGTCCATACATTCTTCCAGTTTCTCTTC |
| GLUT4 F | GTATCATCTCTCAGTGGCTTGGAAG |
| GLUT4 R | TAGGAGGCAGCAGCGTTGG |
R, reverse; F, forward; miR, microRNA; RDX, radixin; LRRC17, leucine rich repeat containing 17; CYP19A1, aromatase; GLUT4, glucose transporter 4.
Western blot assay
First, 200 μL protein lysate (including 2 μL protease inhibitor and 2 μL phosphatase inhibitor) was added to each well. The mixture was stirred well on ice until the cells were completely lysed. A 5× loading buffer containing β-mercaptoethanol was added and mixed, the mixture was boiled for 15 minutes, then stored at −20 °C for use. The extracted protein samples were separated by 10% polyacrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). After blocking with 5% bovine serum albumin (BSA) for 2 h, the membrane was incubated with a primary antibody overnight. Antibodies for LRRC17, RDX, glucose transporter 4 (GLUT4), aromatase (CYP19A), and GAPDH were obtained from Abmart Inc. (Shanghai, China). The next day, the membranes were incubated with the corresponding secondary antibody for 1 h. Finally, immunoreactive bands were visualized by the enhanced chemiluminescence (ECL) method.
Luciferase reporter assay
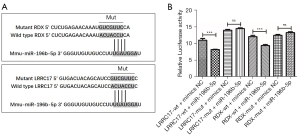
The binding sites of miR-196b-5p were predicted by a bioinformatics website (http://www.targetscan.org). The 293T cells were cultured at a density of (0.3–1)×105 cells/well in 24-well culture plates and transfected with 2μg of dual-luciferase reporter construct pmirGLO-LRRC17/RDX-wt/mut, and co-transfected with 50 nM miR-196b-5p mimics/mimics control using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. At 4 h post-transfection, the transfection medium was removed and replenished with medium containing 10% FBS (Gibco, Rockville, MD, USA). At 48 h post-transfection, luciferase activity was measured using the Dual-Luciferase Reporter Gene Assay Kit (Beyotime, Shanghai, China).
Statistical analysis
All experiments were repeated at least 3 times. The data were expressed as mean ± standard deviation (SD) and analyzed by GraphPad Prism software using the t-test or one-way analysis of variance (ANOVA). P<0.05 was considered to be statistically significant.
Results
Clinical parameters, endocrine parameters, and transcriptome sequencing analysis of VD-deficient mice
To illustrate the effect of VDD, mouse serum 1,25-(OH)2D3 levels were measured by the ELISA method and mouse ovaries were sectioned and stained with H&E. The ovarian sections of the control group showed that the peripheral cortex had a large number of follicles at different developmental stages. The structure of the ovarian medulla was normal (Figure 1A). In contrast, the ovaries obtained from VD-deficient mice showed a distorted shape (Figure 1B). VD-deficient mice (n=5) had 1,25-(OH)2D3 levels of 19.56±1.51 ng/mL compared with control mice (n=5) that had 1,25-(OH)2D3 levels of 62.34±2.70 ng/mL (P<0.01; Figure 1C). For the measurement of ovarian function in the pathology of cyst formation, the levels of serum estradiol, progesterone, and testosterone were evaluated by ELISA. The results showed that the testosterone level of VD-deficient mice was significantly higher than that of the control group, while the estradiol level was significantly lower than that of the control group (P<0.05). However, there was no significant difference in terms of LH and FSH levels between the two groups (P>0.05; Figure 1C).
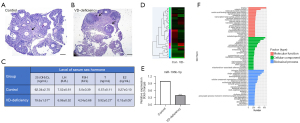
In order to explore the mechanism of 1,25-(OH)2D3 in improving ovarian function, RNA was extracted from the ovaries and sequenced, and the gene expression levels of the VD-deficient group and control group were analyzed. RNA-seq showed that the expression of 6,543 genes changed in VD-deficient ovaries. A total of 672 differentially expressed (DE) miRNAs were identified, while transcripts per million (TPM) values of >4 in both libraries were retained for further analysis (Figure 1D, available online: https://cdn.amegroups.cn/static/public/atm-21-6081-01.xlsx). Among them, 33 miRNAs were reported to have log2-fold change and false discovery rate ≤0.05 in upregulation or downregulation, regardless of miRNA abundance. Furthermore, 17 miRNAs were significantly upregulated while 16 miRNAs were downregulated in the ovarian tissue of VD-mice compared with controls (Table 2, available online: https://cdn.amegroups.cn/static/public/atm-21-6081-02.xlsx). RNA-seq demonstrated that miR-196b-5p was significantly downregulated in the ovaries of VD-deficient mouse models. MiR-196b-5p expression in VD-deficient mouse ovarian tissue was verified via qPCR, which showed significantly lower expression compared with the control group (P<0.001; Figure 1E). These data showed that the transcriptional spectrum changed significantly in the absence of 1,25-(OH)2D3 to change ovarian function.
Table 2
| MiRNA | Target genes |
|---|---|
| Downregulation | Upregulation |
| Novel miRNA-84 | Arfgef1, Adgrb3, Khdrbsz, Aff3, Ankrd44, Vwc2l, Fn1, Tns1, Wdfy1, Dock10 |
| Novel miRNA-7 | Slc13a3, Rbak, Ascc1, Timeless, Inpp46 |
| mmu-miR-200c-5p | Nxph1, Cox10, Ifrd1 |
| mmu-miR-196b-5p | Nsmf, Ctdc1, Katna1, Map3k5, Plazg6, Rpgr |
| Novel miRNA-142 | Zc3hc1, Trip4, Arhgap12 |
| Upregulation | Downregulation |
| mmu-miR-378d | Sntg1, Sgk3, Ppp1r42, Cspp1, A830018L16Rik, Slco5a1, Ncoa2, Eya1, Terf1, Stua2 |
| mmu-miR-669o-5p | Sntg1, Sgk3, Adgr63, Fam168b, Ankrd44, Dpp10, Nckap5, Tem163, Zranb3, Brinp3 |
| Novel miRNA-118 | Cspp1, Adgrb3, Slc39a10, Abi2, Vwc2l, Smarcal1, Pid1, Nckap5, Nav1, Colgalt2 |
| Novel miRNA-54 | Ntm, Afhgap42 |
| mmu-miR-466i-5p | Arhgef4, Cdk15, Zfp142, Clgt1a9, Lrrfip1, Clasp1, Nckap5, Zranb3, Kgl1, Astn1 |
MiRNA, microRNA; VD, vitamin D.
In order to explore the effects of 1,25-(OH)2D3 on the intracellular functions and signaling pathways of ovarian cells, we used Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis to determine the biological functions and signal pathways of DE mRNAs enrichment. DE mRNAs were significantly enriched in biological processes (BPs), including various developmental processes, migration processes, and small molecular catabolism processes (Figure 1F). Among the top 20 items of significant enrichment, 11 items belonged to the BP category [the first 10 items were biological regulation, cellular process, single cell BP, metabolic process, localization, development process, cellular component (CC) tissue or biogenesis, stimulus response, multicellular BP, immune system process], 7 items belonged to the CC category (cell part, organelle, membrane part, membrane part, extracellular region part, macromolecular complex), while two items belonged to the molecular function (MF) category (binding, catalytic activity). Thus, most of the differentially expressed genes (DEGs) were related to cell metabolism and development in BPs.
DE mRNAs also exhibited significant involvement in various pathways, primarily including the PI3K-Akt signal pathway, MAPK signal pathway, HTLV-I infection, RAS signal pathway, endocytosis, proteoglycan in cancer, regulation of actin cytoskeleton, insulin signal pathway, tight junction, and GnRH signal pathway (Table 3, available online: https://cdn.amegroups.cn/static/public/atm-21-6081-03.xlsx).
Table 3
| Pathway ID | Pathway | DEGs with pathway annotation (n=9,509), n (%) | All genes with pathway annotation (n=17,443), n (%) | P value |
|---|---|---|---|---|
| ko04912 | GnRH signaling pathway | 192 (2.02) | 269 (1.54) | 3.85E-09 |
| ko04020 | Calcium signaling pathway | 349 (3.67) | 497 (2.85) | 1.33E-13 |
| ko04014 | Ras signaling pathway | 462 (4.86) | 689 (3.95) | 3.22E-12 |
| ko04310 | Wnt signaling pathway | 258 (2.71) | 400 (2.29) | 1.70E-05 |
| ko04910 | Insulin signaling pathway | 288 (3.03) | 448 (2.57) | 8.81E-06 |
| ko04010 | MAPK signaling pathway | 563 (5.92) | 785 (4.50) | 1.67E-24 |
| ko04911 | Insulin secretion | 207 (2.18) | 285 (1.63) | 7.88E-11 |
| ko04114 | Oocyte meiosis | 221 (2.32) | 306 (1.75) | 4.74E-11 |
| ko04210 | Apoptosis | 156 (1.64) | 254 (1.46) | 1.07E-02 |
| ko04151 | PI3K-Akt signaling pathway | 610 (6.41) | 960 (5.50) | 2.36E-09 |
KEGG, Kyoto Encyclopedia of Genes and Genomes; DEGs, differentially expressed genes.
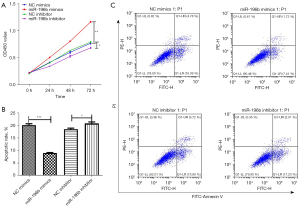
MiR-196b-5p promoted cell proliferation and suppressed cell apoptosis
In order to verify the hypothesis that miR-196b-5p may be involved in GC proliferation, KGN cells were transiently transfected with miR-196b-5p mimics, miR-196b-5p inhibitor, or controls. As illustrated in Figure 2A, the CCK-8 assays revealed that upregulation of miR-196b-5p resulted in a promotion of KGN cell proliferation. Flow cytometry assays revealed that low expression of miR-196b-5p could significantly inhibit KGN cell apoptosis (Figure 2B,2C).
MiR-196b-5p reduced OS and promoted glucose uptake and steroidogenesis
Some studies have shown that apoptosis of various cell types, including GCs, may be related to increased levels of reactive oxygen species (ROS) (20). We determined the effect of miR-196b-5p on the total ROS content. As shown in Figure 3A,3B, miR-196b-5p mimics significantly decreased the amount of ROS in KGN cells compared to NC mimics (P<0.001). In contrast, miR-196b-5p inhibitor treatment increased the ROS in KGN cells compared to negative control (NC) inhibitor treatment (P<0.05). The energy requirement of normal folliculogenesis mainly depends on GC glucose uptake in the surrounding tissue through the GLUT on the cell membrane and the production of pyruvate and lactic acid through glycolysis as the main energy source. Next, we studied the glucose uptake capacity of miR-196b-5p-overexpressed KGN cells. The glucose content in the supernatants of the cells was examined after the cells were cultured for 24, 48, and 72 h. There was no significant difference in glucose content among all groups at 24–48 h. At 72 h, glucose uptake became stronger in the miR-196b-5p mimics group (P<0.01) and weaker in the miR-196b-5p inhibitor group (P<0.05; Figure 3C).
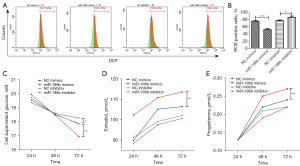
Next, we investigated the effect of miR-196b-5p overexpression on the production of progesterone and estradiol by KGN cells. Cells were incubated with certain concentrations of miR-196b-5p mimics or inhibitor for 24, 48, and 72 h. Secretions of both estradiol (Figure 3D) and progesterone (Figure 3E) were increased by miR-196b-5p mimics in KGN cells at 48–72 h (P<0.01). There were no significant differences in estradiol and progesterone among the miR-196b-5p inhibitor and NC inhibitor groups at 24–72 h.
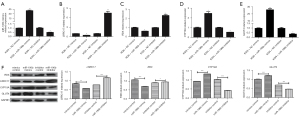
To strengthen these data, we investigated the effect of miR-196b-5p on the mRNA expression of LRRC17, RDX, GLUT4 and CYP19A gene. RT-PCR results identified that the miR-196b-5p expression levels were significantly upregulated in the miR-196b-5p mimics group compared with the NC group (P<0.01). Moreover, miR-196b-5p expression was significantly downregulated in the miR-196b-5p inhibitor group compared with the inhibitor NC group (P<0.05). These results indicated the successful overexpression or knockdown of miR-196b-5p in KGN cells (Figure 4A). As shown in Figure 4B,4C, we found similar effects of miR-196b-5p mimics on mRNA LRRC17 and RDX expression in KGN cells. GLUT4 mRNA expression was significantly increased in the miR-196b-5p mimic group and significantly increased in the inhibitor group (Figure 4D,4E). The results of western blotting are consistent with those of RT-PCR. The expression levels of LRRC17 and RDX were decreased after miR-196b-5p overexpression, while the expression levels of GLUT4 and CYP19A were increased after miR-196b-5p overexpression (Figure 4F). These results further verified that miR-196b-5p directly regulated LRRC17 or RDX and promoted glucose uptake and sex steroid production by GLUT4 and CYP19A in KGN cells.
Prediction of target genes and luciferase reporter assays
In order to study the mechanism of miR-196b-5p in inhibiting the proliferation of GCs, we used TargetScan to find its potential target genes. RDX and LRRC17, which are closely related to cell proliferation, have sparked our interest. To verify the prediction of TargetScan, we constructed a luciferase reporter vector containing predicted RDX and LRRC17 3'UTR binding sites (Figure 5A). The results showed that overexpression of miR-196b-5p resulted in a significant decrease in luciferase activity after co-transfection with RDX or LRRC17 WT 3'UTR, while there was no change in luciferase activity after co-transfection with RDX or LRRC17 MUT 3'UTR (P<0.001, P>0.05; Figure 5B).
Discussion
In this study, a mouse model of VDD was established as previously described (19), and transcriptome sequencing analysis revealed that miR-196b-5p was abnormally downregulated in the ovarian tissues of VD‑deficient mice, which was further confirmed using RT-qPCR of the extracted ovarian tissues from the mice. Functional experiments further demonstrated that overexpression of miR-196b-5p mimics facilitated cell proliferation and inhibited apoptosis in KGN cells. It is reported that increasing the level of miR-196b-5p can be achieved by downregulating type I collagen α 1 chain (COL1A1), which significantly inhibited proliferation and migration of MDA-MB-231 and MDA-MB-468 cells in breast cancer (21). In addition, miR-196b-5p is associated with some pathological changes, such as lung adenocarcinoma (22), hepatocellular carcinoma (23), keloid (24), and colorectal cancer (25), among others. It is also reported that mouse miR-196b can specifically target the 5' noncoding region of insulin 2 splicing isoforms. The RNA binding protein HUD, which inhibits insulin translation, was replaced by miR-196b, indicating that miR-196b upregulates insulin 2 translation (26). They found that increased miR-196b expression induced by glucose may be another mechanism by which glucose stimulates insulin synthesis. The role of miR-196b in ovarian GCs is worthy of further study.
Our study showed that overexpression of miR-196b-5p resulted in a promotion of KGN cell proliferation and low expression of miR-196b-5p could significantly inhibit KGN cell apoptosis. MiR-196b-5p is a positive factor related to the proliferation of ovarian GCs. In addition, RDX and LRRC17 were direct targets of miR‑196b-5p in KGN cells. Overexpression of miR-196b-5p led to significantly decreased luciferase activity by co-transfection with RDX or LRRC17 WT 3'UTR, while there was no change with RDX or LRRC17 MUT 3'UTR. It has been reported that RDX is a direct target of miR‑196b-5p in human gastric cancer (27). Studies (28) have shown that miR-200b regulates the proliferation and invasion of breast cancer cells by targeting RAD expression. The overexpression of miR-200b inhibits the expression of RAD and reduces the proliferation and invasion of breast cancer cells. Chen et al. (29) demonstrated that silencing the expression of RAD in pancreatic cancer cells significantly inhibited cell proliferation and tumorigenicity in vivo. RDX seems to be a positive factor in cell proliferation, but this is not reflected in our experimental results. Decreased LRRC17 has been shown to be a serum risk marker for osteoporotic fracture in the spines of postmenopausal women (30). Song et al. (31) demonstrated that the modulation of the LRRC17 gene may delay or even restore the balance of osteogenic and adipogenic differentiation in autologous bone marrow mesenchymal stem cells derived from patients with idiopathic necrosis of the femoral head (INFH). There are few reports on the function of LRRC17 in ovarian GCs, though so far one study has demonstrated that LRRC17 could be a prognostic gene in ovarian cancer as it regulates cancer cell viability through the p53 pathway (32).
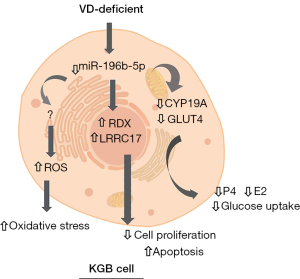
Hyperinsulinemia and IR play a central role in the pathogenesis of PCOS. They can affect the severity of clinical features, though are not associated with the existence of obesity. GLUT4 is a member of the GLUT family, which is preferentially expressed in muscle and adipose tissue. GLUT4 is responsible for insulin-stimulated glucose uptake, which may lead to IR (33). Our study showed that overexpression of miR-196b-5p can significantly reduce the expression of GLUT4, resulting in a decrease in glucose uptake. PCOS is a highly prevalent endocrine-metabolic disorder associated with IR. The decrease of glucose uptake and utilization by skeletal muscle and adipocytes caused by the decrease of GLUT4 expression or activity is an important molecular basis of IR. A prospective cross-sectional study showed that IR secondary to PCOS insulin-mediated decreased glucose uptake and increased insulin secretion was partly due to decreased GLUT4 expression in adipocytes without a compensatory increase in GLUT1 expression (34). The analysis of signaling components of the IRS/PI3K/AKT pathway showed that the expression of GLUT4 was significantly decreased in PCOS patients and the IR control group (35). Their results point to a new mechanism by which miR-93 regulates insulin-stimulated glucose uptake and demonstrates that the upregulated expression of miR-93 in all women with IR with or without PCOS may be the cause of IR in this syndrome. The CYP19A gene responsible for aromatase P450 is located on chromosome 15q21.2 and is necessary for estrogen formation. It is reported that obese and lean women with PCOS have low aromatase activity. Studies have also shown that interruption of the steroid production pathway caused by androgen overdose is a key factor in abnormal follicle formation and dominant follicular selection failure in hyperandrogen-induced PCOS women. The key enzyme for the production of estradiol in the ovaries is CYP19A1, which is involved in the transformation of androgen to estradiol in GCs (36). In our study, secretions of both estradiol and progesterone were increased by miR-196b-5p mimics in KGN cells, as well as the expression of CYP19A. In addition, our study showed miR-196b-5p mimics significantly decreased the amount of ROS in KGN cells. OS is an important inducer of ovarian senescence, which leads to the decline of the reproductive ability of humans and different animals. We showed that miR-196b-5p reduced the ROS content. Unfortunately, we did not detect the expression of ROS-related proteins and the PI3K-Akt signaling pathway associated with IR. In fact, obesity or IR may increase the OS of these cells. It will be interesting to determine whether miR-196b-5p is an effective antioxidant molecule. Recent reports have identified a close interaction between VDD and OS in exacerbating the pathophysiology of PCOS (37,38).
In conclusion, we found that miR-196b-5p was significantly downregulated in the ovaries of VDD mice. In KGN cells, after inhibiting the expression of miR-196b-5p, cell proliferation decreased and apoptosis increased. At the same time, it was found that the decreased expression of CYP19A and GLUT4 led to a decrease in glucose uptake and the secretion of estrogen and progesterone. After inhibiting the expression of miR-196b-5p, the increase of intracellular ROS content indicated the increase of OS. It has been proven that RDX and LRRC17 are the target genes miR-196b-5p (Figure 6). This is a preliminary but pioneering study to elucidate the functions of miR-196b-5p, RDX, and LRRC17 in KGN cells and their relationships. In the meantime, there are some limitations in our study. First, we performed in vitro experiments in KGN cell lines, so we were unable to predict the in vivo effect of miR-196b-5p on ovarian function. Second, a large number of PCOS patients are needed to further verify the expression of miR-196b-5p, RDX, and LRRC17. Therefore, we plan to continue this experiment in our subsequent work.
Acknowledgments
Funding: This study was funded by the Youth Program of Changzhou Health Commission (QN201942), the Young Scientists Foundation of Changzhou No. 2 People’s Hospital (2019K009) and Project of Youth Scientists of Changzhou Health Commission (CZQM2020100).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-6081
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-6081
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-6081). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments in this study were carried out in strict accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People’s Republic of China (11-14-1988). The animal experimental design and protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University (approval number: 2020-62).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018;14:270-84. [Crossref] [PubMed]
- Ajmal N, Khan SZ, Shaikh R. Polycystic ovary syndrome (PCOS) and genetic predisposition: a review article. Eur J Obstet Gynecol Reprod Biol X 2019;3:100060. [Crossref] [PubMed]
- Khan MJ, Ullah A, Basit S. Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. Appl Clin Genet 2019;12:249-60. [Crossref] [PubMed]
- Lerchbaum E, Rabe T. Vitamin D and female fertility. Curr Opin Obstet Gynecol 2014;26:145-50. [Crossref] [PubMed]
- Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol 2012;166:765-78. [Crossref] [PubMed]
- Faghfoori Z, Fazelian S, Shadnoush M, et al. Nutritional management in women with polycystic ovary syndrome: a review study. Diabetes Metab Syndr 2017;11:S429-32. [Crossref] [PubMed]
- Fung JL, Hartman TJ, Schleicher RL, et al. Association of vitamin D intake and serum levels with fertility: results from the Lifestyle and Fertility Study. Fertil Steril 2017;108:302-11. [Crossref] [PubMed]
- Rudick B, Ingles S, Chung K, et al. Characterizing the influence of vitamin D levels on IVF outcomes. Hum Reprod 2012;27:3321-7. [Crossref] [PubMed]
- Rudick BJ, Ingles SA, Chung K, et al. Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil Steril 2014;101:447-52. [Crossref] [PubMed]
- Kikuchi S, Hata M, Fukumoto K, et al. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat Genet 2002;31:320-5. [Crossref] [PubMed]
- Malcuit C, Trask MC, Santiago L, et al. Identification of novel oocyte and granulosa cell markers. Gene Expr Patterns 2009;9:404-10. [Crossref] [PubMed]
- Paul P, Chakraborty A, Sarkar D, et al. Interplay between miRNAs and human diseases. J Cell Physiol 2018;233:2007-18. [Crossref] [PubMed]
- Sirotkin AV, Lauková M, Ovcharenko D, et al. Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J Cell Physiol 2010;223:49-56. [PubMed]
- Tu J, Cheung AH, Chan CL, et al. The role of microRNAs in ovarian granulosa cells in health and disease. Front Endocrinol (Lausanne) 2019;10:174. [Crossref] [PubMed]
- Lee CT, Wang JY, Chou KY, et al. 1,25-dihydroxyvitamin D3 increases testosterone-induced 17beta-estradiol secretion and reverses testosterone-reduced connexin 43 in rat granulosa cells. Reprod Biol Endocrinol 2014;12:90. [Crossref] [PubMed]
- Merhi Z, Doswell A, Krebs K, et al. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab 2014;99:E1137-45. [Crossref] [PubMed]
- Bakhshalizadeh S, Amidi F, Alleyassin A, et al. Modulation of steroidogenesis by vitamin D3 in granulosa cells of the mouse model of polycystic ovarian syndrome. Syst Biol Reprod Med 2017;63:150-61. [Crossref] [PubMed]
- Li P, Xu X, Cao E, et al. Vitamin D deficiency causes defective resistance to Aspergillus fumigatus in mice via aggravated and sustained inflammation. PLoS One 2014;9:e99805. [Crossref] [PubMed]
- Sun H, Shi Y, Shang Y, et al. MicroRNA 378d inhibits Glut4 by targeting Rsbn1 in vitamin D deficient ovarian granulosa cells. Mol Med Rep 2021;23:369. [Crossref] [PubMed]
- Yang H, Xie Y, Yang D, et al. Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget 2017;8:25310-22. [Crossref] [PubMed]
- Zhu X, Rao X, Yao W, et al. Downregulation of MiR-196b-5p impedes cell proliferation and metastasis in breast cancer through regulating COL1A1. Am J Transl Res 2018;10:3122-32. [PubMed]
- Xu Q, Xu Z. miR-196b-5p promotes proliferation, migration and invasion of lung adenocarcinoma cells via targeting RSPO2. Cancer Manag Res 2020;12:13393-402. [Crossref] [PubMed]
- Zhai H, Zhang X, Chen S, et al. RP5-1120P11.3 promotes hepatocellular carcinoma development via the miR-196b-5p-WIPF2 axis. Biochem Cell Biol 2020;98:238-48. [Crossref] [PubMed]
- Yang J, Deng P, Qi Y, et al. NEAT1 knockdown inhibits keloid fibroblast progression by miR-196b-5p/FGF2 axis. J Surg Res 2021;259:261-70. [Crossref] [PubMed]
- Xin H, Wang C, Chi Y, et al. MicroRNA-196b-5p promotes malignant progression of colorectal cancer by targeting ING5. Cancer Cell Int 2020;20:119. [Crossref] [PubMed]
- Panda AC, Sahu I, Kulkarni SD, et al. miR-196b-mediated translation regulation of mouse insulin2 via the 5'UTR. PLoS One 2014;9:e101084. [Crossref] [PubMed]
- Tsai MM, Wang CS, Tsai CY, et al. MicroRNA-196a/-196b promote cell metastasis via negative regulation of radixin in human gastric cancer. Cancer Lett 2014;351:222-31. [Crossref] [PubMed]
- Yuan J, Xiao C, Lu H, et al. miR-200b regulates breast cancer cell proliferation and invasion by targeting radixin. Exp Ther Med 2020;19:2741-50. [Crossref] [PubMed]
- Chen SD, Song MM, Zhong ZQ, et al. Knockdown of radixin by RNA interference suppresses the growth of human pancreatic cancer cells in vitro and in vivo. Asian Pac J Cancer Prev 2012;13:753-9. [Crossref] [PubMed]
- Hong N, Kim BJ, Kim CH, et al. Low plasma level of leucine-rich repeat-containing 17 (LRRc17) is an independent and additive risk factor for osteoporotic fractures in postmenopausal women. J Bone Miner Res 2016;31:2106-14. [Crossref] [PubMed]
- Song D, Wu ZS, Xu Q, et al. LRRC17 regulates the bone metabolism of human bone marrow mesenchymal stem cells from patients with idiopathic necrosis of femoral head through Wnt signaling pathways: a preliminary report. Exp Ther Med 2021;22:666. [Crossref] [PubMed]
- Oh CK, Park JJ, Ha M, et al. LRRC17 is linked to prognosis of ovarian cancer through a p53-dependent anti-apoptotic function. Anticancer Res 2020;40:5601-9. [Crossref] [PubMed]
- Zhang N, Liu X, Zhuang L, et al. Berberine decreases insulin resistance in a PCOS rats by improving GLUT4: dual regulation of the PI3K/AKT and MAPK pathways. Regul Toxicol Pharmacol 2020;110:104544. [Crossref] [PubMed]
- Ezeh U, Chen IY, Chen YH, et al. Adipocyte expression of glucose transporter 1 and 4 in PCOS: Relationship to insulin-mediated and non-insulin-mediated whole-body glucose uptake. Clin Endocrinol (Oxf) 2019;90:542-52. [Crossref] [PubMed]
- Panghiyangani R, Soeharso P. CYP19A1 gene expression in patients with polycystic ovarian syndrome. J Hum Reprod Sci 2020;13:100-3. [Crossref] [PubMed]
- Chen YH, Heneidi S, Lee JM, et al. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 2013;62:2278-86. [Crossref] [PubMed]
- Kyei G, Sobhani A, Nekonam S, et al. Assessing the effect of MitoQ10 and Vitamin D3 on ovarian oxidative stress, steroidogenesis and histomorphology in DHEA induced PCOS mouse model. Heliyon 2020;6:e04279. [Crossref] [PubMed]
- Safaei Z, Bakhshalizadeh SH, Nasr Esfahani MH, et al. Effect of vitamin D3 on mitochondrial biogenesis in granulosa cells derived from polycystic ovary syndrome. Int J Fertil Steril 2020;14:143-9. [PubMed]
(English Language Editor: C. Betlzar)


