Analytical assessment of the novel Maglumi squamous cell carcinoma antigen (SCCA) immunoluminometric assay
Introduction
The generic terms of squamous cell carcinoma antigen (SCCA) identifies two highly homologous proteins (i.e., SCCA1 and SCCA2), which are part of the family of serine protease inhibitors. Both proteins are encoded by two 1,711 bp long, tandemly arranged genes (SCCA1 and SCCA2) on chromosome 18q21.3 (1). SCCA is physiologically produced by the basal and parabasal layers of normal squamous epithelia, but its serum or plasma concentration is considerably enhanced in patients with various forms of squamous cell carcinoma (SCC), especially in those with hepatocellular carcinoma (2), carcinoma of uterine cervix (3), lung carcinoma (4), esophageal cancer (5), breast carcinoma (6), head and neck SCC (7). Although the active role played by SCCA in cancer biology is still uncertain, recent evidence suggests that this protein may strongly impact cancer behaviour by promoting local invasion and metastatization (8).
According to this biological evidence, SCCA is now regarded as one of the most efficient biomarker for staging, monitoring anticancer treatment and predicting the prognosis of SCCs (9), so that its measurement is constantly increasing in clinical laboratories. Therefore, the aim of this study was to evaluate the analytical performance of the novel Maglumi SCCA sandwich immunoluminometric assay on Maglumi 2000.
Materials and methods
Immunoassay characteristics
The Maglumi SCCA sandwich immunoluminometric assay [Shenzhen New Industries Biomedical Engineering Co. (SNIBE), Shenzhen, China] has been developed for use on Maglumi fully automated analyzers series. Briefly, the method uses an anti-SCCA monoclonal antibody labelled with amino-butyl-ethyl-isoluminol (ABEI), a second monoclonal antibody labelled with fluorescein isothiocyanate (FITC) and magnetic microbeads coated with anti-FITC antibodies. A total sample volume of 80 µL is incubated with the reagents and mixed thoroughly. After development of the immunocomplex, a magnetic field is applied to favour magnetic microbeads sedimentation, and the supernatant is removed by extensive washing. The starter reagents are then added to the cuvette, a flash chemiluminescent reaction is initiated and the light signal is finally measured by a photomultiplier as relative light units (RLUs). The recorded optical density (OD) is proportional to the concentration of SCCA present in the test sample. The functional sensitivity and the diagnostic cut-off, of the immunoassay, as for manufacturer’s declaration, are 0.13 and 2.5 ng/mL, respectively.
Analytical evaluation
The present analytical evaluation of Maglumi SCCA was aimed to assess the imprecision, linearity and comparability against a widely used technique. For the intra-assay imprecision, 3 serum pools obtained from routine inpatient samples and displaying low, intermediate and high SCCA values, were measured in 20 consecutive runs. The inter-assay imprecision was assessed by duplicate measurement of 3 additional serum pools obtained from routine inpatient samples and displaying low, intermediate and high SCCA values, for 10 consecutive working days. The results of the imprecision studies were finally reported as coefficient of variation (CV). The linearity of the assay was tested by preparing a pool obtained from routine inpatient serum samples and displaying a high SCCA value (i.e., 18 ng/mL), which was then serially diluted at fixed ratios (1:9; 2:8; 3:7; 4:6; 5:5; 6:4; 7:3, 8:2; 9:1) with a pool also obtained from routine inpatient serum samples but displaying very low SCCA concentration (i.e., 1.0 ng/mL). Each serial dilution was then measured in duplicate and the theoretical values were calculated from the measured values of the undiluted serum pool. Linearity was estimated by means of linear regression analysis and Spearman’s correlation. The comparison study included 100 consecutive inpatient serum samples referred to the local laboratory for routine SCCA assessment, with values covering the most clinically significant range of SCC concentrations (i.e., 0.4–15.2 ng/mL). Results of Maglumi SCCA were compared with those obtained on the same serum samples with a well validated automated immunofluorescence assay (i.e., BRAHMS SCC on BRAHMS Kryptor system, BRAHMS, Hennigsdorf, Germany). According to manufacturer’s specification, the functional sensitivity of Kryptor SCC is 0.34 ng/mL, the diagnostic cut-off is 1.9 ng/mL, the total imprecision is comprised between 0.6–5.2%. The correlation between methods was assessed by means of Deming fit and Spearman’s correlation, whereas the bias and its 95% CI were estimated with Bland-Altman difference plot. The concordance between Maglumi SCCA and Kryptor SCC at the respective diagnostic cut-offs (i.e., 2.5 and 1.9 ng/mL) was calculated by the κ coefficient.
The statistical analysis was performed with Analyse-it (Analyse-it Software Ltd, Leeds, UK). This analytical evaluation was entirely based on pre-existing inpatient serum samples referred for routine SCCA testing, and the material was obtained after analysis was completed. All samples were anonymized prior to Maglumi SCCA assessment, so that no patient informed consent was necessary. The study was carried out in accordance with the Declaration of Helsinki and was approved by the local institutional review Board.
Results
The results of the imprecision study using Maglumi SCCA are shown in Table 1. The intra- and inter-assay imprecision was comprised between 2.6–4.2% and between 5.0–7.3%, respectively. The linearity of the test was excellent in the range of SCCA values comprised between 1.0 and 18.0 ng/mL, as shown by the linear regression analysis obtained by plotting measured and theoretical SCCA concentrations (r=0.998; P<0.001). The results of the comparison study are shown in Figures 1,2. Thirteen out of the 100 samples tested ought to be excluded from the final statistical analysis since they displayed SCCA values below the functional sensitivity of Kryptor SCC (i.e., <0.34 ng/mL), so that a final number of 87 serum samples was used for statistical analysis. A highly significant correlation was observed between Maglumi SCCA and Kryptor SCC in the range of values comprised between 0.44 and 15.18 ng/mL (r=0.960; P<0.001). The equation of Deming fit was (Maglumi SCCA) = 1.15 × (Kryptor SCC) + 0.58 (Figure 1). The mean bias, as calculated by means of Bland-Altman plot analysis, was 0.79 ng/mL (95% CI, 0.61-0.97 ng/mL) (Figure 2). The strength of agreement between Kryptor SCC and Maglumi SCCA was 95% at the respective diagnostic cut-offs (κ, 0.85; 95% CI, 0.71–0.99; P<0.001).
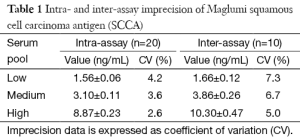
Full table
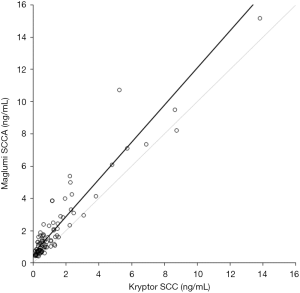
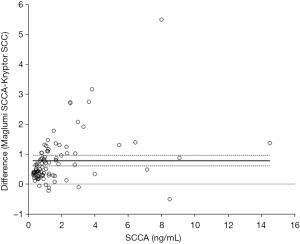
Discussion
The demand for routine measurement of SCCA is rapidly increasing in clinical laboratories, due to the central role that this biomarker plays in staging and monitoring patients with various forms of SCCs, especially in those with poorly differentiated and advanced metastatic cancers (10). Besides manual enzyme linked immunometric sandwich assays (ELISAs), only one automated immunoassay has been available for routine assessment of SCCA in the past few years, that is BRAHMS Kryptor SCC. More recently, however, the Chinese company SNIBE has developed a new sandwich immunoluminometric assay, which has complemented the large panel of cancer biomarkers already available on Maglumi fully automated analyzers series. The results of our analytical evaluation attest that this novel method is characterized by excellent imprecision (Table 1), optimal linearity, and is appropriately correlated with the previously available Kryptor SCC automated immunoassay. In particular, the low inter-assay CV, which was found to be always <7.3%, would make this method particularly suitable for longitudinal monitoring of cancer patients. The broad linearity of the assay also enables to cover the most frequently observed, and clinically meaningful, SCCA concentrations that may be encountered during staging and monitoring of cancer patients. Despite we also observed acceptable correlation (r=0.960) and agreement at the respective diagnostic thresholds (95%) between Kryptor SCC and Maglumi SCCA, a meaningful bias emerged, thus implying that the two techniques cannot be used interchangeably, as previously reported for most cancer biomarker assays (11).
Conclusions
The results of this study confirm that Maglumi SCCA may be regarded as a suitable alternative to BRAHMS Kryptor SCC for routine and fully-automated assessment of SCC in clinical laboratories.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Suminami Y, Kishi F, Sekiguchi K, et al. Squamous cell carcinoma antigen is a new member of the serine protease inhibitors. Biochem Biophys Res Commun 1991;181:51-8. [PubMed]
- Montagnana M, Danese E, Lippi G. Squamous cell carcinoma antigen in hepatocellular carcinoma: Ready for the prime time? Clin Chim Acta 2015;445:161-6. [PubMed]
- Sheng X, Du X, Zhang X, et al. Clinical value of serum HMGB1 levels in early detection of recurrent squamous cell carcinoma of uterine cervix: comparison with serum SCCA, CYFRA21-1, and CEA levels. Croat Med J 2009;50:455-64. [PubMed]
- Song WA, Liu X, Tian XD, et al. Utility of squamous cell carcinoma antigen, carcinoembryonic antigen, Cyfra 21-1 and neuron specific enolase in lung cancer diagnosis: a prospective study from China. Chin Med J (Engl) 2011;124:3244-8. [PubMed]
- Chen M, Huang J, Zhu Z, et al. Systematic review and meta-analysis of tumor biomarkers in predicting prognosis in esophageal cancer. BMC Cancer 2013;13:539. [PubMed]
- Catanzaro JM, Guerriero JL, Liu J, et al. Elevated expression of squamous cell carcinoma antigen (SCCA) is associated with human breast carcinoma. PLoS One 2011;6:e19096. [PubMed]
- Snyderman CH, D'Amico F, Wagner R, et al. A reappraisal of the squamous cell carcinoma antigen as a tumor marker in head and neck cancer. Arch Otolaryngol Head Neck Surg 1995;121:1294-7. [PubMed]
- Murakami A, Fukushima C, Yositomi K, et al. Tumor-related protein, the squamous cell carcinoma antigen binds to the intracellular protein carbonyl reductase. Int J Oncol 2010;36:1395-400. [PubMed]
- Gadducci A, Tana R, Cosio S, et al. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature. Crit Rev Oncol Hematol 2008;66:10-20. [PubMed]
- Wright GM, Russell PA. The promise of lung master protocol for squamous cell carcinoma: one trial to rule them all, one trial to find them…? Ann Transl Med 2015;3:219. [PubMed]
- Pritzker KP. Cancer biomarkers: easier said than done. Clin Chem 2002;48:1147-50. [PubMed]


