
Modified microvessel density based on perfusion distance: a preferable NSCLC prognostic factor
Introduction
Blood perfusion is crucial for tumorigenesis and progression (1-4). Furthermore, vascular remodeling can improve drug delivery (5). Thus, blood perfusion profile-based biomarkers, including microvessel density (MVD) and microvessel area (MVA), have emerged as mainstream markers of patient survival and outcomes, and may be predictive of patient prognosis (6-11). However, their prognostic value in terms of patient survival in various types of solid tumors (12,13), including non-small cell lung cancer (NSCLC), has been controversial (7,9,14-16) . This is not unexpected because the quantity of microvessels is only one of many decisive parameters reflecting perfusion efficiency. In addition to MVD and MVA, blood perfusion efficiency is based on vessel diffusion distance, blood flow rate, and resistance to blood perfusion in the extracellular matrix (ECM) (17), especially in desmoplastic tumors (18,19).
In most solid tumors, cancer cell niches are surrounded by a rich desmoplastic stroma, which separates cancer cells from microvessels (3,18,20). The diffusion and convection of nutrients and oxygen crossing the stroma to the cancer cells was found to be impaired by matrix-fibro and high interstitial fluid pressure caused by the dense extracellular matrix (18,20). Elongated distances can result in significant deficiencies in perfusion of the tumor (17,21,22). Therefore, microvessels near cancer niches likely allow superior perfusion compared to those far away from cancer cells. Our previous study determined the diffusion distances between microvessels and their nearest cancer cells, defined as microvessels near cancer cells, Dmvcc. Long distances were shown to be strongly associated with poor survival (23). However, Dmvcc cannot accurately reflect the MVD. Herein, we hypothesized that the density of microvessels near cancer cells may be more representative of the real perfusion system and a more powerful prognostic factor compared with MVD.
In this study, a modified MVD (mMVD) value was defined as the density of microvessels within the optimal cutoff of Dmvcc (23). The mMVD was shown to be a preferable prognostic prediction marker for NSCLC patients compared to total MVD. The following article is presented in accordance with the REMARK reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-6566/rc).
Methods
Patients and samples
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by institutional ethics committee of Zhejiang Cancer Hospital (No. IRB-2017-67). Individual patient consent for this study was waived due to the retrospective nature of this investigation.
This retrospective study included a total of 100 patients who underwent surgical resection for the treatment of NSCLC at Zhejiang Cancer Hospital (Hangzhou, China) between July 2011 and October 2012. The inclusion criteria were as follows: (I) the patients were histologically diagnosed with primary NSCLC; (II) the patients underwent surgical resection and had available tumor tissue of the primary lesion; (III) the patients with full clinicopathologic information. All clinicopathological characteristics and survival outcome data were available. Tumor stage, histology, and differentiation classifications were performed in accordance with the tumor, node, and metastasis (TNM) system or the World Health Organization (WHO) criteria (24). The overall survival (OS) was calculated from the day of diagnosis until death or the last follow-up. Progression-free survival (PFS) was recorded from the day of diagnosis until evidence of recurrence was observed. Tumor recurrence was monitored using abdominal computed tomography (CT), abdominal ultrasonography, magnetic resonance imaging (MRI), and chest X-ray examinations. The last data of follow-up was July 20th, 2016.
Immunohistochemistry
Paraffin-embedded tumor tissues were consecutively cut into 5 µm thick sections and processed for immunohistochemical staining using the endothelial cell marker CD31, as described previously (23). Briefly, Antigen retrieval was carried out using the EnVisionTM FLEX Target Retrieval Solution (pH =9.0, Dako), followed by incubation with a rabbit polyclonal CD31 antibody (dilution: 1:300, Proteintech, Rosemont, USA) at 4 °C overnight. Sections were then incubated with the secondary antibody (PV-9000, Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for 1 hour, followed by color development with 3,3’-diaminobenzamine in Tris-HCl (50 mmol/L, pH =7.5) containing 0.005% hydrogen peroxide, and counterstaining with hematoxylin.
Assessment of Dmvcc, tMVD, and mMVD
The immunohistochemically stained tumor tissue sections were observed under a microscope at 100× magnification. According to a previously described method (25,26), the field showing the most intense vascularization was selected as the “hotspot”. Two experienced investigators measured the Dmvcc as the distance from each microvessel to its nearest cancer cell within the hotspot, at a 200× magnification. The optimal Dmvcc cutoff value for prognostic prediction was calculated using Cutoff Finder (27), an online software developed by the Translational Tumor Research Team at the Institute of Pathology, Charité-Universitätsmedizin Berlin (version 2.1, January 8, 2013, available at https://molpathoheidelberg.shinyapps.io/CutoffFinder_v1/). Subsequently, the number of total microvessels or microvessels within the cutoff-Dmvcc from the cancer niche was determined as tMVD or mMVD, respectively, as previously described (25,26).
Statistical analysis
Statistical analyses were conducted using SPSS software (Version 23.0, IBM Inc., New York, USA), Cutoff Finder (27) (Version 2.1, Institute of Pathology, Berlin, Germany) and GraphPad Prism software (version 7.00, GraphPad Software, La Jolla, CA, USA). Categorical data are expressed as counts and percentages, while continuous data are expressed as median values and ranges. Where suitable, Student’s t-test, and linear regression analysis were performed. To evaluate the strength of the prognostic prediction of Dmvcc, tMVD, and mMVD, each possible cutoff was investigated separately and a Cox proportional hazard model was fitted to each of the corresponding groups of patients. The corresponding hazard ratios (HRs) were plotted for all cutoff points. The optimal cutoff point was defined as the point with the most significant split (log-rank test). Hazard ratios (HRs), including 95% confidence intervals (CIs), were calculated. Patients were divided into groups based on tMVD and mMVD, and the survival curves were computed using the Kaplan-Meier method and compared using the log-rank test. Multivariate analysis was performed using the Cox regression model to assess the predictive potential of each factor independent of other clinical characteristics. A two-tailed P value <0.05 was considered statistically significant.
Results
Patients
A total of 100 patients, including 74 males and 26 females, with a median age of 59 years (range: 40–79 years) were included in this study (Table 1). The majority of patients (71/100) presented with early-stage NSCLC (stages I and II). The differentiation status was classified into 3 categories, namely, poorly differentiated (n=49), moderately differentiated (n=47), and well-differentiated (n=1). There were 54 cases of adenocarcinomas and 43 cases of squamous carcinomas. The median follow-up time was 51.1 months (range: 45.5–60.0 months). At the end of the follow-up period, 29 (29%) patients died. Eight patients were lost to follow-up and 52.2% of the remaining patients (48/92) experienced recurrence.
Table 1
| Clinicopathologic variables | Number (%) | Dmvcc (μm)† | MVD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dmvcc | P value ‡ | tMVD | P value ‡ | mMVD | P value ‡ | P value§ | |||
| Age (years) | |||||||||
| <60 | 51 (51.0) | 25.3±5.5 | 0.768 | 39.1±2.3 | 0.413 | 25.6±2.3 | 0.647 | <0.0001*** | |
| ≥60 | 49 (49.0) | 23.4±3.8 | 41.7±2.3 | 27.1±2.4 | <0.0001*** | ||||
| Gender | |||||||||
| Male | 74 (74.0) | 27.4±4.3 | 0.133 | 40.8±1.8 | 0.639 | 26.2±1.9 | 0.891 | <0.0001*** | |
| Female | 26 (26.0) | 15.9±3.4 | 39.1±3.4 | 26.7±3.2 | 0.0098 | ||||
| Smoking history | |||||||||
| Never | 33 (33.0) | 17.8±3.4 | 0.168 | 39.7±3.0 | 0.775 | 27.1±2.7 | 0.753 | 0.0028 | |
| Prior or current | 67 (67.0) | 27.6±4.7 | 40.7±1.9 | 26.0±2.1 | <0.0001*** | ||||
| Disease stage | |||||||||
| Early (stage I & II) | 71 (71.0) | 23.9±4. 5 | 0.834 | 43.4±2.0 | 0.003** | 28.8±2.116 | 0.019* | <0.0001*** | |
| Advanced (stage III & IV) | 29 (29.0) | 25.5±3.6 | 33.0±2.0 | 20.34±1.895 | <0.0001*** | ||||
| Tumor histology | |||||||||
| Adenocarcinoma | 54 (54.0) | 17.2±2.7 | 0.052 | 37.7±2.1 | 0.134 | 25.89±1.879 | 0.455 | <0.0001*** | |
| Squamous | 43 (43.0) | 33.7±6.8 | 44.1±2.5 | 27.67±2.983 | <0.0001*** | ||||
| Others | 3 (3.0) | 20.2±9.3 | 37.0±4.4 | 15.67±2.333 | 0.0125 | ||||
| Tumor differentiation | |||||||||
| Poorly | 49 (49.0) | 23.6±3.0 | 0.887 | 38.8±2.0 | 0.525 | 25.8±2.187 | 0.524 | <0.0001*** | |
| Moderately | 47 (47.0) | 22.7±5.8 | 42.0±2.7 | 27.94±2.603 | 0.0003*** | ||||
| Well | 1 (1.0) | 153.0 | 50.0 | 11.0 | |||||
| 24.4±3.3 | 40.4±1.59 | 26.4±1.64 | <0.0001*** | ||||||
†, Dmvcc represents the distance from microvessels to the nearest cancer cell; ‡, intra-group difference; §, difference between tMVD and mMVD; *, P<0.05; **, P<0.01; ***, P<0.001. tMVD, total microvessel density; mMVD, modified microvessel density based on perfusion distance.
Dmvcc profile
The value of Dmvcc in the tumor samples varied from 1.61–269.75 µm, with a median value of 13.10 µm. No significant differences in Dmvcc values were observed between subpopulations stratified by age (P=0.768), gender (P=0.133), smoking history (P=0.168), disease stage (P=0.834), histology (P=0.052), nor differentiation status (P=0.887). The optimal Dmvcc cutoff value for prognosis was determined to be 20 µm.
tMVD and mMVD profiles
Representative images of tMVD are presented in Figure 1A. The median values for tMVD and mMVD were 38 (range: 17–81) and 23 (range: 2–70), respectively (Table 1 and Figure 1B). Patients presenting with different disease stages showed significantly different tMVD (P=0.003) and mMVD values (P=0.019) (Table 1 and Figure 1C). However, when subpopulations were stratification by age, gender, smoking history, histology, or differentiation status, there were no significant differences among the tMVD (P=0.134–0.775) nor mMVD values (P=0.455–0.891). The mMVD values were 2.6–98.6% of the tMVD values, with a median ratio of 72.5%, which was remarkably lower than that of tMVD, and was independent of clinicopathological characteristics.
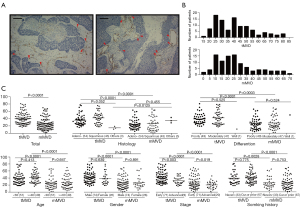
Prognostic strength and significance of the mMVD and tMVD values
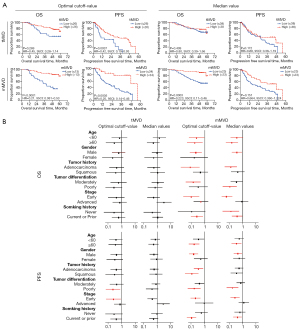
The strength and significance of the tMVD and the mMVD for prognostic stratification were demonstrated using both receiver operating characteristic (ROC) curves and significance test plots (Figure 2A). The area under the ROC curve (AUC) for OS analysis was 0.56 for tMVD (with sensitivity of 81.2% and specificity of 34.5%) and 0.74 for mMVD (sensitivity of 87.3% and specificity of 51.7%). In contrast, the AUC values for PFS analysis were comparable (0.63 and 0.66 for tMVD and mMVD, respectively). Only 2.9% (1 out of 34) and 27.3% (9 out of 33) of the investigated tMVD cutoff points were significantly correlated with OS and PFS, respectively. In contrast, 80% and 58.8% of the mMVD cutoff points were significantly correlated with OS and PFS, respectively. The optimal cutoff values of mMVD for OS and PFS were determined as 13 and 34, respectively, while those of tMVD were 26.5, for both OS and PFS (Figure 2B).

Univariate survival analysis
To further evaluate the prognostic value of MVD, the population was stratified based on the optimal MVD cutoff or median MVD. As shown in Figure 3A, the tMVD was not a satisfactory prognostic predictor for OS, either dichotomized by the optimal cutoff value (HR =0.45, 95% CI: 0.28 to 1.14, P=0.269) or the median value (HR =0.80, 95% CI: 0.39 to 1.66, P=0.409). Instead, the mMVD was a promising prognostic predictor of OS, either dichotomized by the optimal cutoff value (HR =0.21, 95% CI: 0.081 to 0.53, P<0.0001) or the median value (HR =0.22, 95 % CI: 0.11 to 0.46, P=0.0003). The PFS was significantly correlated with both the optimal cutoff value of mMVD (HR =0.25, 95% CI: 0.14 to 0.46, P=0.0005) and the tMVD (HR =0.42, 95% CI: 0.19 to 0.93, P=0.0037). However, no significant correlations with either the median value of mMVD (HR =0.66, 95% CI: 0.36 to 1.22, P=0.151) nor the tMVD value (HR =0.68, 95% CI: 0.38 to 1.19, P=0.172) were observed. Moreover, the strength of the mMVD in survival prediction was verified in subgroup survival analysis with most clinicopathological characteristics (Figure 3B).
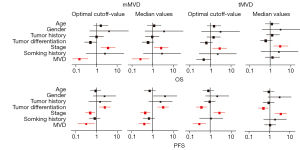
Multivariate survival analysis
Cox’s proportional hazard estimation was conducted to assess the independent prognostic values of tMVD and mMVD for survival outcomes. As shown in Figure 4, higher mMVD, regardless of either cut-off or median setting, robustly predicted both longer OS (cutoff value: HR =0.16, 95% CI: 0.07 to 0.37, P<0.001; median value: HR =0.26, 95% CI: 0.10 to 0.67, P=0.005) and PFS (cutoff value: HR =0.31, 95% CI: 0.12 to 0.80, P=0.013; median value: HR =0.40, 95% CI: 0.21 to 0.76, P=0.005), and this was independent of other clinicopathologic characteristics. In contrast, tMVD was not an independent predictor of disease-related death (cutoff value: HR =0.47, 95% CI: 0.21 to 1.05, P=0.066; median value: HR =1.30, 95% CI: 0.58 to 2.92, P=0.530) nor recurrence (median value: HR =1.02, 95% CI: 0.55 to1.91, P=0.946), Interestingly, patients with higher tMVD stratified by optimized cutoff were associated with longer PFS (HR =0.29, 95% CI: 0.14 to 0.59, P=0.001). In addition, advanced stage of disease independently predicted a higher risk of disease-related mortality and recurrence.
Discussion
Blood perfusion, as a major contributor to tumor progression, has a profound influence on nutrient supply, oxygen diffusion, and drug delivery in solid tumors. Therefore, biological factors based on the intratumor blood perfusion system, including MVD and MVA, have been theoretically proposed as potential prognostic markers (17). Nevertheless, to date, none have been proven to be a reliable and intrinsic prognostic marker across different types of solid tumors (17). This may be partly attributed to the fact that they are not absolute determinant factors of intratumor blood perfusion, and at least the distance from microvessels to cancer niches plays a crucial role in perfusion deficiency, but was is not considered in evaluation of MVD or MVA. The present study identified complex contributors to perfusion deficiency, including vascular density and perfusion distance. The results revealed that the mMVD of a perfusion distance-confined sub-cluster of microvessels, which is in close proximity to cancer cells (within 20 µm), was inversely correlated with OS and PFS. Moreover, it was shown to have greater prognostic prediction power compared to tMVD.
Blood perfusion efficiency is determined by multiple factors, including microvessel density, vessel diffusion distance, blood flow rate, and resistance to blood perfusion in the ECM (17). In particular, in tumor tissue desmoplastic stroma between cancer-niches and microvessels, the delivery of oxygen, nutrients, and drugs can be significantly hampered by a thick ECM and high-levels of interstitial fluid pressure (IFP) (18,19). Therefore, a short Dmvcc is considered fundamental for efficient perfusion (17). For instance, the oxygen partial pressure (pO2) is inversely proportional to the square of the perfusion distance (28). Data from breast cancer xenografts showed that pO2 was decreased by approximately 40% and 100% at distances of 50 and 70 µm from the vessels, respectively (29,30). Furthermore, hypoxia (pO2 <50% of vascular oxygen pressure) was observed at a distance of less than 50 µm in NSCLC specimens (31). Glucose concentration has been reported to decrease by 40% at a diffusion distance of 100 µm (29). Despite the meaningful impact of delivery distance on the perfusion efficiency of tumors, the perfusion profile data of patients are difficult to evaluate. Therefore, delivery distance was introduced as a surrogate marker. In this current study, the Dmvcc of NSCLC patients varied from 1.6 to 270 µm, and this was significantly correlated with the survival outcome. A Dmvcc of 20 µm was determined to be the cutoff distance for prognosis. For the rich desmoplastic stroma in patient tumors, the cutoff distance was shorter than that obtained from xenograft data. Thus, 20 µm was used as the cutoff value to distinguish microvessels near cancer cells from those far away from cancer cells. The microvessels near the cancer niches (within 20 µm) were counted and defined as mMVD. As a result, approximately 75% of the total microvessels were close to the cancer niche.
The prognostic values of mMVD and tMVD were evaluated and compared in three aspects: strength of prediction determined using ROC analysis and significance test with continuous data, univariate survival analysis using the log-rank test, and multivariate survival analysis using Cox regression with variously dichotomized data. As a more comprehensive perfusion-based factor to tMVD, mMVD represented not only the quantity but also the perfusion distance profiles of microvessels and was presented as a more powerful prognostic factor. Compared with tMVD, mMVD was a superior prognostic factor, especially for OS, with a higher AUC value (0.74 vs. 0.56), a larger fraction of OS-predictable-MVD (80% vs. 2.9%), and a meaningful role in univariate, subgroup, or multivariate analysis with either an optimal cutoff value or median value.
Interestingly, in this cohort of NSCLC patients, low-level mMVD and tMVD was associated with poor survival. To date, the prognostic value of tMVD remains controversial. Although tMVD was shown to be a detrimental prognostic factor in a pooled meta-analysis of 35 studies (7), its prognostic value was dismissed in a 17-center study for NSCLC (16). Moreover, positive correlations between low-tMVD and poor survival have been reported in other solid tumors, including esophageal (14) and ovarian cancers (15). This may be attributed to the fact that lower mMVD induces poorer blood perfusion and results in a higher numbers of hypoxia-susceptible tumor cells, which is a prime motivator of tumor progression via numerous pathways including apoptosis-resistance, immune escape, and chemoresistance (31-33). In line with this, substantial evidence indicates that poorly vascularized solid cancer regions are hypoxic and more likely to be resistant to chemotherapy and radiotherapy (14,34).
These results demonstrated that a complex factor involving microvessel quantity and perfusion distance may be a favorable prognostic marker for NSCLC patients. However, there were certain limitations to this study. In the absence of data on perfusion distance and perfusion efficiency in patients, the cutoff distance was determined according to patient survival. Therefore, the cutoff value of Dmvcc should be examined in other tumors to verified the results. Further investigations regarding the reliability and applicability of the cutoff value in various types of solid tumors should be conducted. In addition, there are several other potential factors may influence perfusion, such as microenvironment. For example, specific subtype of perivascular like cells in microenvironment of NSCLC can promote vascular leakage (35). The macrophages expressing tie2, which regulated by hypoxia inducible factor α subunits, affect tumor perfusion in rat breast cancer (36). These factors were not included in analyses of this study. Finally, several factors may be associated with the prognosis of NSCLC, such as the tumor differentiation and TNM stage, et al. In our study, the mMVD was found significantly different in different group of TNM stage. Also, we analyzed tMVD and mMVD values in patients classified according to various other clinicopathological characteristics. The mutation status of driver genes and related targeted therapy may also affect the outcome of NSCLC. However, all patients analyzed in this study were hospitalized in from 2011 to 2012, the detection of driver genes was not prevalent at that time. We failed to collect the signature mutations of these patients retrospectively and the relationship with mMVD. Further research may be carried out in the future.
In summary, this report demonstrated that mMVD can act as a prognostic marker of OS and PFS in patients with NSCLC. It presented superior sensitivity, specificity, and clinical applicability compared to tMVD. A complex consideration of vascular intensity and the diffusion distance from vessels to cancer cells by introducing the definition of a modified MVD might provide novel insights into neovascularization-based prognostic predictions.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of China (Grant numbers: 81773819 and 81973396 to Luo Fang, and 81803585 to Like Zhong); the Natural Science Foundation of Zhejiang Province (Grant number: Y16H160129 to Luo Fang, LYY18H310006 to Like Zhong, and LQ17H300001 to Yu Song); the Science and Technology in Zhejiang Province Chinese Medicine Program (Grant number: 2015ZA036 to Luo Fang); the Medical Science Research Foundation of Zhejiang Province (Grant number: 2019KY473 to Zheng Shi, and 2013KYA027 to Luo Fang); and Key Laboratory of Clinical Cancer Pharmacology and Toxicology Research of Zhejiang Province (2020E10021).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-6566/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-6566/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-6566/coif). YS reports that this work was supported by the Natural Science Foundation of Zhejiang Province (Grant number: LQ17H300001). LZ reports that this work was supported by the Natural Science Foundation of China (Grant numbers: 81803585) and the Natural Science Foundation of Zhejiang Province (Grant number: LYY18H310006). ZS reports that this work was supported by the Medical Science Research Foundation of Zhejiang Province (Grant number: 2019KY473). LF reports that this work was supported by the Natural Science Foundation of China (Grant numbers: 81773819 and 81973396), the Natural Science Foundation of Zhejiang Province (Grant number: Y16H160129), the Science and Technology in Zhejiang Province Chinese Medicine Program (Grant number: 2015ZA036), and the Medical Science Research Foundation of Zhejiang Province (Grant number: 2013KYA027). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by institutional ethics committee of Zhejiang Cancer Hospital (No. IRB-2017-67). Individual patient consent for this study was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Macchiarini P, Fontanini G, Hardin MJ, et al. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet 1992;340:145-6. [Crossref] [PubMed]
- Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: prognostic and therapeutic implications. J Clin Oncol 2005;23:3243-56. [Crossref] [PubMed]
- Kim E, Stamatelos S, Cebulla J, et al. Multiscale imaging and computational modeling of blood flow in the tumor vasculature. Ann Biomed Eng 2012;40:2425-41. [Crossref] [PubMed]
- Zhang T, Nie J, Liu X, et al. Correlation Analysis Among the Level of IL-35, Microvessel Density, Lymphatic Vessel Density, and Prognosis in Non-Small Cell Lung Cancer. Clin Transl Sci 2021;14:389-94. [Crossref] [PubMed]
- Gouarderes S, Mingotaud AF, Vicendo P, et al. Vascular and extracellular matrix remodeling by physical approaches to improve drug delivery at the tumor site. Expert Opin Drug Deliv 2020;17:1703-26. [Crossref] [PubMed]
- Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer 2002;86:1566-77. [Crossref] [PubMed]
- Meert AP, Paesmans M, Martin B, et al. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 2002;87:694-701. [Crossref] [PubMed]
- Korkolopoulou P, Thymara I, Kavantzas N, et al. Angiogenesis in Hodgkin's lymphoma: a morphometric approach in 286 patients with prognostic implications. Leukemia 2005;19:894-900. [Crossref] [PubMed]
- Koster A, van Krieken JH, Mackenzie MA, et al. Increased vascularization predicts favorable outcome in follicular lymphoma. Clin Cancer Res 2005;11:154-61. [PubMed]
- Tong YH, He Y, Hu LY, et al. Elevated proportion of collapsed microvessels indicate poor survival outcome in patients with non-small cell lung cancer. Tumori 2019;105:494-500. [Crossref] [PubMed]
- Fang L, He Y, Liu Y, et al. Adjustment of Microvessel Area by Stromal Area to Improve Survival Prediction in Non-Small Cell Lung Cancer. J Cancer 2019;10:3397-406. [Crossref] [PubMed]
- Dong Y, Ma G, Liu Y, et al. Prognostic Value of Microvessel Density in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Dis Markers 2020;2020:8842795. [Crossref] [PubMed]
- Ntellas P, Dadouli K, Perivoliotis K, et al. Microvessel Density and Impact of Angiogenesis on Survival of Resected Pancreatic Cancer Patients: A Systematic Review and Meta-analysis. Pancreas 2019;48:233-41. [Crossref] [PubMed]
- Zhang SC, Hironaka S, Ohtsu A, et al. Computer-assisted analysis of biopsy specimen microvessels predicts the outcome of esophageal cancers treated with chemoradiotherapy. Clin Cancer Res 2006;12:1735-42. [Crossref] [PubMed]
- Gadducci A, Viacava P, Cosio S, et al. Intratumoral microvessel density, response to chemotherapy and clinical outcome of patients with advanced ovarian carcinoma. Anticancer Res 2003;23:549-56. [PubMed]
- Trivella M, Pezzella F, Pastorino U, et al. Microvessel density as a prognostic factor in non-small-cell lung carcinoma: a meta-analysis of individual patient data. Lancet Oncol 2007;8:488-99. [Crossref] [PubMed]
- Menon C, Fraker DL. Tumor oxygenation status as a prognostic marker. Cancer Lett 2005;221:225-35. [Crossref] [PubMed]
- Ariffin AB, Forde PF, Jahangeer S, et al. Releasing pressure in tumors: what do we know so far and where do we go from here? A review. Cancer Res 2014;74:2655-62. [Crossref] [PubMed]
- Stylianopoulos T, Martin JD, Snuderl M, et al. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer Res 2013;73:3833-41. [Crossref] [PubMed]
- Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release 2015;201:78-89. [Crossref] [PubMed]
- Baish JW, Stylianopoulos T, Lanning RM, et al. Scaling rules for diffusive drug delivery in tumor and normal tissues. Proc Natl Acad Sci U S A 2011;108:1799-803. [Crossref] [PubMed]
- Kim M, Gillies RJ, Rejniak KA. Current advances in mathematical modeling of anti-cancer drug penetration into tumor tissues. Front Oncol 2013;3:278. [Crossref] [PubMed]
- Ding H, Sun J, Song Y, et al. Long Distance From Microvessel to Cancer Cell Predicts Poor Prognosis in Non-Small Cell Lung Cancer Patients. Front Oncol 2021;11:632352. [Crossref] [PubMed]
- Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer (UICC) TNM Classification of Malignant Tumors. Oxford, UK: Wiley-Blackwell; 2009.
- Vermeulen PB, Gasparini G, Fox SB, et al. Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer 1996;32A:2474-84. [Crossref] [PubMed]
- Fang L, He Y, Tong Y, et al. Flattened microvessel independently predicts poor prognosis of patients with non-small cell lung cancer. Oncotarget 2017;8:30092-9. [Crossref] [PubMed]
- Budczies J, Klauschen F, Sinn BV, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One 2012;7:e51862. [Crossref] [PubMed]
- Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 1955;9:539-49. [Crossref] [PubMed]
- Vaupel P. Oxygenation of human tumors. Strahlenther Onkol 1990;166:377-86. [PubMed]
- Dewhirst MW. Concepts of oxygen transport at the microcirculatory level. Semin Radiat Oncol 1998;8:143-50. [Crossref] [PubMed]
- Swinson DE, Jones JL, Richardson D, et al. Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small-cell lung cancer. J Clin Oncol 2003;21:473-82. [Crossref] [PubMed]
- Chouaib S, Noman MZ, Kosmatopoulos K, et al. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene 2017;36:439-45. [Crossref] [PubMed]
- Rapisarda A, Melillo G. Overcoming disappointing results with antiangiogenic therapy by targeting hypoxia. Nat Rev Clin Oncol 2012;9:378-90. [Crossref] [PubMed]
- Bhattacharya A, Tóth K, Mazurchuk R, et al. Lack of microvessels in well-differentiated regions of human head and neck squamous cell carcinoma A253 associated with functional magnetic resonance imaging detectable hypoxia, limited drug delivery, and resistance to irinotecan therapy. Clin Cancer Res 2004;10:8005-17. [Crossref] [PubMed]
- Bichsel CA, Wang L, Froment L, et al. Increased PD-L1 expression and IL-6 secretion characterize human lung tumor-derived perivascular-like cells that promote vascular leakage in a perfusable microvasculature model. Sci Rep 2017;7:10636. [Crossref] [PubMed]
- Steinberger KJ, Forget MA, Bobko AA, et al. Hypoxia-Inducible Factor alpha Subunits Regulate Tie2-Expressing Macrophages That Influence Tumor Oxygen and Perfusion in Murine Breast Cancer. J Immunol 2020;205:2301-11. [Crossref] [PubMed]


