
Prevalence and clinical characteristics of hepatitis B surface antigen-negative/hepatitis B core antibody-positive patients with detectable serum hepatitis B virus DNA
Introduction
Hepatitis B virus (HBV) infection is a global public health threat that causes considerable liver-related morbidity and mortality. More than two billion people worldwide have been infected with HBV (1), with 80 million people infected in China (2). Delayed treatment of HBV infection can lead to liver cirrhosis and even liver cancer (3).
Serological marker assays are the primary form of laboratory auxiliary diagnosis for HBV infections (4). Serological markers of HBV include hepatitis B virus surface antigen (HBsAg), hepatitis B surface antibody (HBsAb), hepatitis B e antigen (HBeAg), hepatitis B e antibody (HBeAb), and hepatitis B core IgM and IgG antibodies (HBcAb) (5). Currently, the methods for clinical laboratory detection of HBV serological markers mainly include enzyme-linked immunosorbent assays (ELISAs) and chemiluminescent microparticle immunoassays (CMIAs). However, because the detection of serological markers is not sensitive to HBV replication, it can only determine whether there is an HBV infection. In contrast, the quantitative detection of HBV DNA can directly and flexibly monitor the replication of HBV DNA in the serum, thereby clearly distinguishing between the different stages of infection (6). However, the quantitative detection of HBV DNA, which can only be performed in a PCR laboratory, requires advanced equipment and experienced technicians. Therefore, this method has not gained much traction in the clinical space.
Notably, HBV DNA has been detected in HBsAg-negative/HBcAb-positive individuals and this can pose a safety risk to blood transfusions and organ transplantations. Additionally, it can affect the monitoring of patients with HBV infections and their treatment (7-9).
In the general population, accurate epidemiological data regarding the HBV DNA status of HBsAg-negative/HBcAb-positive patients are of great significance for the prevention of HBV. However, since China is a large country with differences in economic development, HBV risk factors, and detection techniques across different regions, the prevalence of HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA also varies tremendously across the country. To date, there have been few reports on the prevalence of HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA in central China. In certain populations, such as immunosuppressed patients, HBV may be activated and active viral replication can accelerate liver damage, and even liver failure and death (10,11). Therefore, quantitative detection of HBV DNA to monitor HBV replication is crucial in HBsAg-negative/HBcAb-positive patients treated with immunosuppressive therapy.
This study estimated the prevalence of HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA in the Hunan province by quantifying serum HBV DNA levels using CMIAs. The correlation between viral load and HBV serological markers, liver function indicators, and coagulation function indicators were explored to provide a comprehensive and in-depth diagnostic basis for clinical application. Furthermore, the effects of immunosuppressive therapy on DNA replication were analyzed in HBsAg-negative/HBcAb-positive patients. To the best of our knowledge, our study is the first to evaluate hepatitis B viral load in combination with the HBV serological characteristics of patients, their infection history, and their immunosuppressive therapy. This report will provide valuable information for the improvement of existing testing strategies and the establishment of novel laboratory detection methods for assessing HBV.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/atm-21-6272).
Methods
Patients
Between January and December 2019, 7018 patients were recruited at the Second Xiangya Hospital (Changsha, Hunan, China) and screened for serum levels of HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb. A total of 2,013 HBsAg-negative/HBcAb-positive serum samples were selected and 108 patients had detectable serum HBV DNA. A further 108 patients with matching serotype, but undetectable serum HBV DNA were selected as the control group.
All blood samples were stored at −20 °C for further analyses. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Second Xiangya Hospital Institutional Review Board (IRB number: 2021-108) and written informed consent was obtained from all patients.
Assessment of HBV serological markers
Serum levels of HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb were detected using the Abbott Architect Immunoassay kit (Abbott Diagnostics, Abbott Park, IL, USA) according to the manufacturer’s instructions.
Measurement of serum HBV DNA levels
A 200 µL aliquot of serum was used to extract HBV DNA using a Hepatitis B Viral DNA Quantitative Fluorescence Diagnostic Kit (Sheng Xiang Bio Inc., Changsha, Hunan, China) as per the manufacturer’s protocol and quantified by real-time polymerase chain reaction (RT-qPCR) with an ABI 7000 real-time detection system (Applied Biosystems, Foster City, CA, USA).
Liver biochemical assays
The concentrations of serum albumin, total protein, alanine transaminase (ALT), and aspartate transaminase (AST) were determined using the ARCHITECTc8000 System (Abbott Laboratories, Irving, TX, USA).
Coagulation function index assays
Coagulation function was assessed using the STA-R automatic coagulometer in accordance with the manufacturer’s protocol. The coagulation method was used to detect the prothrombin time (PT), activated partial thromboplastin time (APTT), thromboplastin time (TT), and fibrinogen (Fib). The chromogenic substrate method was used to detect antithrombin III (AT III) levels.
Statistical analysis
Normally distributed data are expressed as mean ± standard deviation (
Patient and public involvement
Patients of the public were directly involved in the present study.
Results
Basic demographic data of the included patients
A total of 7,018 patients who were tested for HBV DNA from January to December 2019 were screened for HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb. Figure 1 summarizes the general workflow of this study. Patients who were HBsAg positive or HBcAb negative, patients presenting with hepatitis C infection, and patients with incomplete clinical data were excluded. Finally, a total of 2,013 HBsAg-negative/HBcAb-positive patients who satisfied the selection criteria were included in this study and serum HBV DNA was detected in 108 patients, resulting in a prevalence of 5.4% (108/2,013).
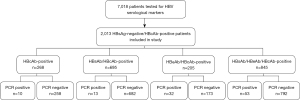
Prevalence of HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA and viral load in different serological groups
The 2,013 HBsAg-negative/HBcAb-positive patients were classified according to different serological markers. The prevalence of serum HBV DNA varied depending on HBV serological subtypes. Furthermore, viral load was also significantly different depending on serological subtypes (Table 1).
Table 1
| HBV serology status | Number of total patients (n) | Number of patients with detectable serum HBV DNA (%) | HBV viral load (IU/mL) |
|---|---|---|---|
| HBsAb/HBcAb-positive | 695 | 13 (1.87) | 7.47 (4.82, 22.15)*# |
| HBsAb/HBeAb/HBcAb-positive | 845 | 53 (6.27) | 27.74 (5.64, 78.82) |
| HBeAb/HBcAb-positive | 205 | 32 (15.61) | 43.5 (23.34, 149.75)#,& |
| HBcAb-positive | 268 | 10 (3.73) | 3.45 (2, 81.6) |
*, P<0.05, HBsAb/HBcAb-positive patients compared to the HBsAb/HBeAb/HBcAb-positive patients; #, P<0.05, HBsAb/HbcAb-positive patients compared to the HbeAb/HbcAb-positive patients; &, P<0.05, HBeAb/HBcAb-positive patients compared to the HBsAb/HBeAb/HBcAb-positive patients. HBV, hepatitis B virus; HBsAb, hepatitis B surface antibody; HBcAb, hepatitis B core antibody; HBeAb, hepatitis B e antibody.
Baseline characteristics of patients with detectable serum HBV DNA compared with the control group
A total of 108 HBsAg-negative/HBcAb-positive patients with undetectable serum HBV DNA, who were matched by age, gender, and serological characteristics, were recruited as the control group. Table 2 shows the clinical data of patients with detectable serum HBV DNA and patients with undetectable serum HBV DNA. The serum levels of ALT, AST, and AT III were significantly lower in patients with undetectable HBV DNA compared to patients with detectable HBV DNA (P<0.05).
Table 2
| Variables | HBV DNA detectable patients (N=108) | HBV DNA undetectable patients (N=108) | P values |
|---|---|---|---|
| Age (years), mean ± SD | 57±15.4 | 56.8±12 | 0.385 |
| Male gender, n (%) | 73 (68.87) | 73 (68.87) | |
| HBV serological profile, n | |||
| HBcAb-positive | 8 | 8 | |
| HBsAb/HBcAb-positive | 25 | 25 | |
| HBeAb/HBcAb-positive | 34 | 34 | |
| HBsAb/HBeAb/HBcAb-positive | 41 | 41 | |
| Liver profile | |||
| Albumin (g/L), mean ± SD | 35.4±7.2 | 35.2±6.1 | 0.892 |
| Total protein (g/L), mean ± SD | 62.7±9.2 | 61.9±8.3 | 0.484 |
| Alanine aminotransferase (U/L), median (IQR) | 21.6 (13.5, 43.5) | 18.1 (12.6, 29.1) | 0.008* |
| Aspartate transaminase (U/L), median (IQR) | 25.0 (17.5, 42.4) | 19.9 (15.2, 26.3) | 0.01* |
| Coagulation profile, mean ± SD | |||
| APTT (second) | 19.1±4.1 | 18±3.5 | 0.45 |
| TT (second) | 12.5±2.2 | 11.6±3.2 | 0.678 |
| PT (second) | 13.5±2.7 | 13.6±2.6 | 0.82 |
| FIB (g/L) | 334.5±10.4 | 313.5±12.5 | 0.09 |
| AT III (g/L) | 89.5±18.8 | 95.2±15.2 | 0.04* |
| Clinical characteristics, n | |||
| Without immunosuppressive | 69 | 72 | |
| With immunosuppressive | 39 | 36 |
Data were presented as median (IQR). *, P<0.05. HBV, hepatitis B virus; HBsAb, hepatitis B surface antibody; HBcAb, hepatitis B core antibody; HBeAb, hepatitis B e antibody; APTT, activated partial thromboplastin time; TT, thrombin time; PT, prothrombin time; FIB, fibrinogen; AT III, antithrombin III.
Correlation of serum levels of ALT, AST, and AT III with viral load in patients with detectable serum HBV DNA
The above results suggested that serum levels of AT III, AST, and ALT may be correlated with viral load in patients with detectable serum HBV DNA. Linear regression analyses were performed using the log10 HBV DNA value as the dependent variable and the three enzymes as independent variables. The log10 HBV DNA value was positively correlated with serum concentrations of ALT (r=0.2309; P<0.05; Figure 2) and AST (r=0.2230; P<0.05; Figure 3) in patients with detectable serum HBV DNA. However, the log10 HBV DNA value was negatively correlated with AT III concentrations (r=0.386; P<0.01; Figure 4).
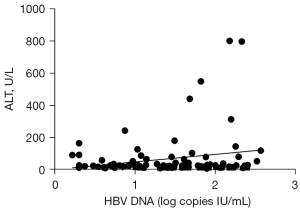
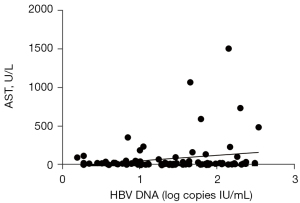
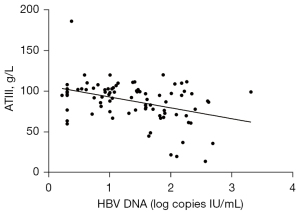
Clinical categories of patients with detectable serum HBV DNA
Patients with detectable serum HBV DNA and patients with undetectable serum HBV DNA were further grouped according to whether they received immunosuppressive therapy. The viral replication, liver function, and coagulation function were assessed. As shown in Table 3, among patients with detectable serum HBV DNA, the ALT and AST levels of patients receiving immunosuppressive therapy were higher than those who did not receive immunosuppressive therapy. However, in patients with undetectable serum HBV DNA, there were no significant differences in ALT nor AST levels between patients who received immunosuppressive therapy and those who did not (Table 4).
Table 3
| Total HBV DNA detectable patients (n=108) | With immunosuppressive therapy (n=39) | Without immunosuppressive therapy (n=69) |
|---|---|---|
| ALT (U/L) | 24 (11.4, 52.6) | 20.9 (13.9, 31.6)* |
| AST (U/L) | 26.8 (17.8, 65.4) | 22.2 (16.8, 33.9)* |
| AT III (g/L) | 93 (79.1, 103.7) | 96.6 (77, 102.2) |
Data were presented as median (IQR). *, P<0.05, patients with immunosuppressive therapy compared to patients without immunosuppressive therapy. HBV, hepatitis B virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AT III, antithrombin III.
Table 4
| Total HBV DNA undetectable patients (n=108) | With immunosuppressive therapy (n=45) | Without immunosuppressive therapy (n=63) |
|---|---|---|
| ALT (U/L) | 15.3 (7.2, 38.7) | 14.2 (13.1, 30.2) |
| AST (U/L) | 20.1 (17.3, 32.5) | 18.6 (16.7, 67.8) |
| AT III (g/L) | 85 (68.1, 140.7) | 94.5 (75.1, 113.1) |
Data were presented as median (IQR). HBV, hepatitis B virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AT III, antithrombin III.
The 108 patients with detectable serum HBV DNA were further grouped according to HBeAb-negative or HBeAb-positive presentation. The serum levels of ALT and AST were significantly higher in HBeAb-positive patients compared to HBeAb negative patients, regardless of the administration of immunosuppressive therapy (Table 5).
Table 5
| Total HBV DNA detectable patients (n=108) | With immunosuppressive therapy (n=39) | Without immunosuppressive therapy (n=69) | |||
|---|---|---|---|---|---|
| HBeAb-positive (n=30) | HBeAb-negative (n=9) | HBeAb-positive (n=43) | HBeAb-negative (n=26) | ||
| ALT (U/L) | 48.2 (16.2, 937.1) | 19.8 (11.3, 37.4)* | 21.8 (13.7, 46.5) | 18 (12.8, 23.1)# | |
| AST (U/L) | 39.1 (20, 388.7) | 25.7 (17.6, 56)* | 25.7 (19.1, 78.8) | 20.9 (12.4, 25.6)# | |
| AT III (g/L) | 86.9±22.6 | 98.6±15 | 85.82±19.27 | 100.2±11.9# | |
*, P<0.05, patients with HBeAb-positive compared to patients with HBeAb-negative in patients with immunosuppressive therapy; #, P<0.05, patients with HBeAb-positive compared to patients with HBeAb-negative in patients without immunosuppressive therapy. Data were presented as median (IQR). HBV, hepatitis B virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AT III, antithrombin III; HBeAb, hepatitis B e antibody.
Discussion
HBsAg is often used as an index for monitoring and evaluating HBV infection and the antiviral treatment response (12). Therefore, the detection of HBsAg is of great significance in the screening, diagnosis, and evaluation of HBV infection and treatment efficacy. Nevertheless, the absence of HBsAg in the blood does not mean that there is no HBV infection. HBcAb is generally regarded as indicative of a previous infection. It often exists in acute or chronic HBV infections and individuals who have recovered from infection (13). Serum HBcAb levels are significantly increased in HBV patients with immune tolerance, low replication, or immune clearance. Furthermore, HBcAb concentrations are positively correlated with the severity of liver damage (14). Therefore, HBsAg-negative/HBcAb-positive patients are more likely to have HBV DNA detected their blood compared to patients with HBsAg-negative serology alone. However, the prevalence of HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA varies significantly among populations worldwide.
Using fluorescent quantitative PCR, this study identified a 5.4% prevalence of HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA in Hunan province, China. Many factors influence the prevalence, such as HBV risk factors in the population and DNA detection methods (15-18). HBV DNA integration may also affect the prevalence. HBV DNA integration have effects on the virus itself and host hepatocytes, effective integration of HBV DNA into the host genome increases the chance of HBV infection in HBsAg-negative patients (19). In Italy, Hong Kong, and the United Kingdom, the prevalence is 11%, 6.9%, and 0%, respectively. In China mainland, there is a significant difference in the prevalence of HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA between developing and developed regions.He et al. reported that the prevalence of HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA in Handan City, Hebei province (developing area) was ten times higher than that recorded in Shanghai (developed area) (20).
During the early stages of HBV infection, different serological markers will manifest due to differences in the immune function of the population, but patients who were negative for multiple serological markers showed positive rates of HBV DNA, indicating that quantitative HBV DNA detection of HBV infection can well supplement the quantitative serological markers of hepatitis B. Therefore, the combined HBV DNA quantitative assay with quantitative serological markers can improve the accuracy of clinical diagnosis of HBV and provide an effective reference basis for clinical judgment of the degree of HBV infection and infectiousness. The prevalence of detectable serum HBV DNA was studied in patients with different HBV serological types. The HBeAb/HBcAb positive group had the highest prevalence of patients with detectable serum HBV DNA and the highest active virus replication. The HBsAb/HBeAb/HBcAb positive serological group had a lower prevalence of patients with detectable serum HBV DNA and a lower viral load compared to the HBeAb/HBcAb positive group, suggesting that HBsAb had a protective effect on the body. HBsAb is usually indicative of immunity after HBV infection. In some developed countries, blood is considered safe when HBsAb reaches a specific titer (21). In HBsAg-negative/HBcAb-positive patients, when HBeAb is positive, both the prevalence of patients with detectable serum HBV DNA (15.61%) and the viral load (43.5 IU/mL) are at the highest levels. When HBsAb is positive at the same time, the viral load decreases (27.74 IU/mL), indicating that viral replication is active when HBeAb is positive, and when the body produces the protective antibody HBsAb, viral replication is inhibited. Many studies have reported that individual HBcAb-positive patients may have a higher prevalence of HBV infection (22), however, in our study, the prevalence was the lowest among the four serological groups, and viral replication was not active. These discrepancies may be due to the small number of such patients selected in this study and the small sample size.
This study demonstrated that HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA had significantly higher serum ALT and AST levels compared to patients with undetectable serum HBV DNA. ALT and AST are the most commonly used liver function indicators. These enzymes are distributed mainly in hepatocytes and increases in serum ALT and AST concentrations are a direct measure of the degree of liver cell damage.
When HBV replicates in HBsAg-negative/HBcAb-positive patients, ALT and AST can rise rapidly, indicating that liver function is impaired at this time. A lot of studies suggested that HBV DNA viraemia levels correlate positively with the inflammatory grade, fibrosis stage and ALT levels (23). Concurrently, AT III concentrations were significantly lower in HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA compared to patients with undetectable serum HBV DNA. Since AT III, which is an essential component in the anticoagulation system, is synthesized by the liver (24), the levels of this enzyme correspondingly decrease during liver dysfunction. Further analysis revealed that in HBsAg-negative/HBcAb-positive patients, HBV DNA viral load was positively correlated with ALT and AST concentrations, and negatively correlated with AT III concentrations. Madan also found that there is significant positive correlation of HBV DNA levels with histological severity of disease and with ALT levels (23). The above results suggest that clinicians should monitor the replication of HBV DNA in HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA and determine liver damage by assessing the concentrations of serum ALT, AST, and AT III, so as to administer the optimal treatment regimen in a timely manner.
In HBsAg-negative/HBcAb-positive patients, immunosuppression can increase the risk of HBV infection (25). In HBsAg-negative/HBcAb-positive patients with undetectable serum HBV DNA, there was no significant difference in liver function nor coagulation function between patients who received immunosuppressive therapy and those who did not. However, in HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA, there were significant differences in liver function and coagulation function indexes in the immunosuppressive treatment group compared to the non-immunosuppressed group (P<0.05), suggesting that liver function was impaired. A retrospective analysis showed that the prevalence of HBV reactivation in patients receiving anti-TNFα therapy for glomerulonephritis was up to 3.62% in serological status of HBsAg- negative/HBcAb-positive (26), which demonstrated that when the immune system of the patient is suppressed by chemotherapy or immunosuppressive therapy, the hidden HBV virus in the body can become active again and begin to replicate, as manifested by an increase in the viral load detected by HBV DNA levels. Therefore, it is recommended that patients receiving immunosuppressive therapy or dialysis therapy should regularly monitor the serum HBV DNA levels if their serotype is HBsAg-negative/HBcAb-positive/HBeAb-positive (27,28).
In summary, although the prevalence of HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA is not high, for patients receiving immunosuppressive therapy, blood transfusion, or transplant patients, the risk of liver function damage caused by reactivated replication of the virus after immunity reduction is greater than that of the normal population. Therefore, clinicians should be aware of the qualitative results of other HBV antigens or antibodies in such individual with HBsAg-negative serology and comprehensively assess viral activity in the body. Furthermore, clinicians should pay attention to the immune function of patients with HBsAg-negative/HBcAb-positive presentation. In cases where HBV-DNA cannot be detected in a timely manner, changes in serum concentrations of ALT, AST, and AT III can be used to speculate on the replication status of the virus, so as to reduce the risk of HBV infection and transmission in these patients.
There were some limitations to this study. First, the number of detectable serum HBV DNA patients with HBsAg-negative/HBcAb-positive was relatively small. Additional studies with larger cohorts are needed to confirm these findings. Second, this investigation only observed the use of immunosuppression in patients with positive HBV DNA. Patients with kidney transplantation, hemodialysis, and anti-tumor drugs may also experience the same decline in immunity. Whether the replication of HBV in these patients is consistent with that shown in this study requires further investigation. Finally, this report only included patients in Hunan, it remains unclear whether these findings are applicable to patients in other regions.
Conclusions
The prevalence of HBsAg-negative/HBcAb-positive patients with detectable serum HBV DNA in Hunan province is 5.4%. In HBsAg-negative/HBcAb-positive patients, HBV DNA detection should be conducted in patients who are on immunosuppressants or those with particular HBV serological presentation. If HBV DNA cannot be detected, clinicians should assess changes in serum ALT, AST, and AT III concentrations to speculate on viral replication status, so as to reduce the risk of HBV infection and transmission.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-6272
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-6272
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-6272). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Second Xiangya Hospital Institutional Review Board (IRB number: 2021-108) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nguyen MH, Wong G, Gane E, et al. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev 2020;33:e00046-19. [Crossref] [PubMed]
- Tout I, Loureiro D, Mansouri A, et al. Hepatitis B surface antigen seroclearance: Immune mechanisms, clinical impact, importance for drug development. J Hepatol 2020;73:409-22. [Crossref] [PubMed]
- European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012;57:167-85. [Crossref] [PubMed]
- Seto WK, Lo YR, Pawlotsky JM, et al. Chronic hepatitis B virus infection. Lancet 2018;392:2313-24. [Crossref] [PubMed]
- Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet 2014;384:2053-63. [Crossref] [PubMed]
- Raimondo G, Locarnini S, Pollicino T, et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol 2019;71:397-408. [Crossref] [PubMed]
- Lee J, Park JY, Huh KH, et al. Rituximab and hepatitis B reactivation in HBsAg-negative/ anti-HBc-positive kidney transplant recipients. Nephrol Dial Transplant 2017;32:722-9. [Crossref] [PubMed]
- Mikulska M, Nicolini L, Signori A, et al. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive allogeneic haematopoietic stem cell transplant recipients: risk factors and outcome. Clin Microbiol Infect 2014;20:O694-701. [Crossref] [PubMed]
- Rios-Ocampo WA, Cortes-Mancera F, Olarte JC, et al. Occult hepatitis B virus infection among blood donors in Colombia. Virol J 2014;11:206. [Crossref] [PubMed]
- Pattullo V. Prevention of Hepatitis B reactivation in the setting of immunosuppression. Clin Mol Hepatol 2016;22:219-37. [Crossref] [PubMed]
- Chen MH, Chen MH, Chou CT, et al. Low but Long-lasting Risk of Reversal of Seroconversion in Patients With Rheumatoid Arthritis Receiving Immunosuppressive Therapy. Clin Gastroenterol Hepatol 2020;18:2573-2581.e1. [Crossref] [PubMed]
- Cachinho JB, François M, Stefic K, et al. Antigène HBs positif en l’absence de toute infection par le virus de l’hépatite B: la face cachée de l’Ag HBs. Ann Biol Clin (Paris) 2019;77:543-8. [Crossref] [PubMed]
- Ramezani A, Banifazl M, Eslamifar A, et al. Serological pattern of anti-HBc alone infers occult hepatitis B virus infection in high-risk individuals in Iran. J Infect Dev Ctries 2010;4:658-61. [Crossref] [PubMed]
- Caviglia GP, Olivero A, Ciancio A, et al. Analytical and clinical evaluation of a novel assay for anti-HBc IgG measurement in serum of subjects with overt and occult HBV infection. Diagn Microbiol Infect Dis 2020;96:114985. [Crossref] [PubMed]
- Alavian SM, Miri SM, Hollinger FB, et al. Occult Hepatitis B (OBH) in Clinical Settings. Hepat Mon 2012;12:e6126. [Crossref] [PubMed]
- Huang X, Hollinger FB. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat 2014;21:153-62. [Crossref] [PubMed]
- Mak LY, Wong DK, Pollicino T, et al. Occult hepatitis B infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J Hepatol 2020;73:952-64. [Crossref] [PubMed]
- Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis 2002;2:479-86. [Crossref] [PubMed]
- Tu T, Budzinska MA, Shackel NA, et al. HBV DNA Integration: Molecular Mechanisms and Clinical Implications. Viruses 2017;9:75. [Crossref] [PubMed]
- He CS, Ma CY, Lu ZC. The analysis of occult hepatitis B virus infection with serum of HBcAb positive and HBsAg negative using Chemiluminescence Immunoassay. Labeled Immunoassays and Clinical Medicine 2019;26:130-3.
- Mabunda N, Zicai AF, Ismael N, et al. Molecular and serological characterization of occult hepatitis B among blood donors in Maputo, Mozambique. Mem Inst Oswaldo Cruz 2020;115:e200006. [Crossref] [PubMed]
- Wang Q, Klenerman P, Semmo N. Significance of anti-HBc alone serological status in clinical practice. Lancet Gastroenterol Hepatol 2017;2:123-34. [Crossref] [PubMed]
- Madan K, Batra Y, Jha JK, et al. Clinical relevance of HBV DNA load in patients with chronic hepatitis B infection. Trop Gastroenterol 2008;29:84-90. [PubMed]
- Allingstrup M, Wetterslev J, Ravn FB, et al. Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 2016;42:505-20. [Crossref] [PubMed]
- Huang YH, Hsiao LT, Hong YC, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol 2013;31:2765-72. [Crossref] [PubMed]
- Fang J, Li W, Peng X, et al. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients receiving immunosuppressive therapy for glomerulonephritis: a retrospective analysis. Int Urol Nephrol 2017;49:475-82. [Crossref] [PubMed]
- Harigai M, Mochida S, Mimura T, et al. A proposal for management of rheumatic disease patients with hepatitis B virus infection receiving immunosuppressive therapy. Mod Rheumatol 2014;24:1-7. [Crossref] [PubMed]
- Winthrop KL, Calabrese LH. Let the fog be lifted: screening for hepatitis B virus. before biological therapy. Ann Rheum Dis 2011;70:1701-3. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


