Effects of methylene blue in acute lung injury induced by oleic acid in rats
Introduction
Under physiological conditions, the pulmonary endothelium forms a semipermeable barrier against the surrounding tissue and controls the dynamics of the exchange of fluid and macromolecules between the blood and the interstitium (1). The permeability of the pulmonary endothelium plays a vital role in the regulation of normal tissue homoeostasis and the maintenance of lung function (2).
Acute lung injury (ALI) is a term used to describe the response to direct or indirect injury to the lungs. In ALI, rupture of the alveolar-capillary barrier determines the protein-rich fluid influx into alveolar spaces (3), and in the process of resolving ALI, this fluid must be reabsorbed (4) Intravenous infusion of oleic acid (OA) in rats acutely causes diffuse alveolar oedema and intra-alveolar hemorrhagic foci and serves as an outstanding model of ALI induction (5-8).
Previous studies have reported that methylene blue (MB) attenuates the aforementioned injuries and exerts a protective effect in lung tissue and reduces the oedema present in ALI as a result of sepsis; MB produces its effect by inhibiting the enzyme soluble guanylate cyclase (sGC), an activator of the nitric oxide/cyclic guanosine 3'–5' monophosphate (NO/cGMP) pathway (9-11).
This study was conducted to enhance our understanding of how sGC inhibition by MB potentially affects pulmonary capillary permeability through its effect on the NO/cGMP pathway. The primary objective was to determine the effect of MB as a prophylaxis and treatment of pulmonary oedema in ALI, and MB was administered before and after inducing lung injury in rats by using OA.
Methods
Animals
Male Wistar rats (200–300 g) used in this study were obtained from the Central Animal Facility of the Campus of Ribeirão Preto, University of São Paulo (USP). The animals were housed under a controlled temperature (22–25 °C) and a 12:12-h light/dark cycle and allowed free access to water and food. All protocols conformed to the guidelines of the Ethics Committee on Animal Experimentation (CETEA), Ribeirão Preto Faculty of Medicine (FMRP).
Experimental design
All animals were premedicated with an intraperitoneal injection of urethane (2 mg/kg). Arterial and venous access was obtained by drying and cannulating the left femoral artery and vein. After arterial blood samples were collected, the animals were sacrificed by exsanguination.
The animals were randomly divided into five groups: (I) Sham, infused with a saline bolus; (II) MB, infused with MB for 2 h; (III) OA, infused with an OA bolus; (IV) MB/OA, infused with MB for 2 h, and at 5 min after the beginning, concurrently infused with an OA bolus; and (V) OA/MB, infused with an OA bolus, and after 2 h, infused with MB for 2 h. After 4 h, blood, bronchoalveolar lavage (BAL), and lung tissue were collected from all groups for analysis of plasma and tissue NO, calculation of the wet weight to dry weight ratio (WW/DW), and histological examination of lung tissue.
Induction of acute lung injury (ALI)
For ALI induction, the animals received an intravenous (iv) infusion of 70 mg/kg of OA (Sigma Chemical Company, St. Louis, MO, USA) in a bolus through the left femoral vein. The control group received saline instead of OA.
Treatment with methylene blue (MB)
Following ALI induction, the animals of the treatment groups were administered an iv infusion of a 3-mg/kg MB bolus followed by infusion of 3 mg/kg/h of MB (Sigma Chemical Company) through the left femoral vein by using an infusion pump (Syringe Infusion Pump, Harvard Apparatus, South Natick, MA, USA). The infusion time was 2 h.
Collection of arterial blood samples
Blood samples were collected, placed in a heparinized tube (1:20), and centrifuged (2,500 rpm, 4 °C, 15 min) to separate the plasma. The plasma sample was transferred to a polypropylene tube that was placed immediately in liquid nitrogen and the sample was then stored in a freezer (−70 °C) for subsequent determination of nitrite/nitrate (NOx).
Collection of bronchoalveolar lavage (BAL)
After the animals were sacrificed, the chest was opened, and the trachea and lungs were dissected and removed en bloc and cut nearly 2 cm above the carina. For BAL collection, a tracheal suction probe was inserted selectively in the left lung to instil1 mL of sodium chloride (NaCl, 0.9%) and aspirate liquid by using a 2-mL hypodermic syringe. Half the instilled volume was collected for analysis, and the sample was stored (−70 °C) for subsequent determination of total protein.
Indirect determination of NO through measurement of nitrite (NO2−) and nitrate (NO3−) and plasma and tissue (NOx)
The upper lobe of the right lung was dissected and the sample was placed in a polypropylene tube, frozen immediately in liquid nitrogen, and then stored in a freezer (−70 °C) for subsequent determination of NOx. Plasma and lung tissue NO levels were measured indirectly by determining serum nitrite and nitrate concentrations by using a Sievers NO Analyser 280Â (Sievers, Boulder, CO, USA).
Analysis of wet lung weight to dry weight ratio
The lower lobe of the right lung was dissected and weighed on an analytical balance (accurate to 0.001 g) to maintain the wet weight. The sample was then placed in a drying oven and sterilised for 60 °C for 24 h and weighed again to obtain the dry weight. The obtained weights were used to calculate the WW/DW values.
Histological analysis
The middle lobe of the right lung was collected and cut longitudinally. A 2-mm-thick central slice was fixed in 10% (v/v) buffered formalin for 24 h and then progressively dehydrated [from alcohol at low concentration of absolute alcohol: (v/v) 50%, 70%, 80%, 90%, 95%, and 100%], and this was followed by leaf clearing in xylene and embedding in paraffin. Next, 3-mm sections were cut using a microtome (Microtome 2040, Reichert-Jung, Germany), placed on slides, and stained with hematoxylin/eosin (HE) for histological analysis by using light microscopy.
Morphometric analysis
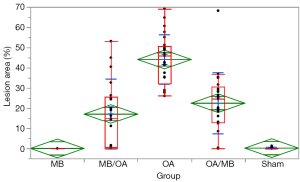
Morphometric analyses were performed by a researcher who was blinded to the protocol design (CTS); for the analyses, the researcher used Leica QWin software (Leica Microsystems Image Solutions, Cambridge, UK) in conjunction with a Leica DMR microscope (Leica, Microsystems GmbH, Wetzlar Germany), a video camera (Leica Microsystems Ltd, Heebrugg, Switzerland), and an online computer. To evaluate ALI, we determined the surface density of the oedema by using the optical density measured in the image analysis (Leica QWin software). The thresholds for oedema were established for each slide after enhancing the contrast to a point at which the oedema could be readily identified as pink fluid. For the analysis, 5 randomly selected, non-coincident fields were measured in 5 animals from each group, at 200× magnification across a total area of 1.25 mm2.
Statistical analysis
Data are expressed as median and the range between the 25% and 75% quartiles. Statistical analysis was carried out with nonparametric (Wilcoxon, Mann-Whitney) tests, and results were considered statistically significant when P<0.05. Also, a comparison between experimental groups with the Sham group was carried out using the Steel method, followed the adjusted power for each group (It is considered as significant alpha of 5%). The statistical analysis and the graphs were performed with the aid of the software JMP 12 for Mac (SAS Institute, Cary, NC, USA).
Results
Pulmonary permeability
The proteins in BAL and the relative value WW/DW were increased in the OA group. MB treatment reduced both BAL proteins and WW/DW values when administered before and after OA; however, any group showed a statistically significant reduction in protein leakage into BAL or WW/DW (Figures 1,2).
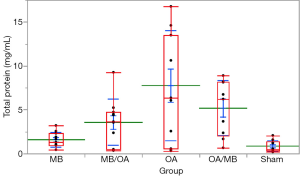
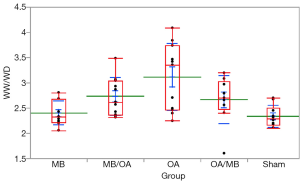
Tissue and plasma (NOx)
Tissue NOx levels were increased in the OA group. MB treatment both before and after OA administration reduced NOx to control levels (Figure 3). However, the difference was not statistically significant. Moreover, no difference in plasma NOx levels was observed (Figure 3).

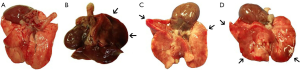
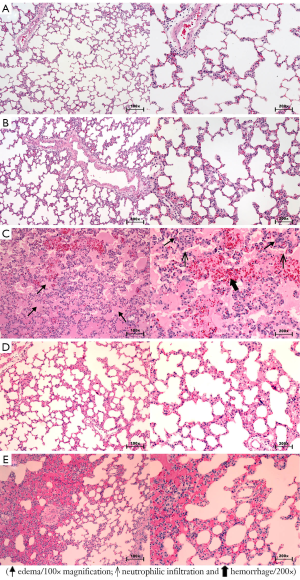
Macroscopic and microscopic changes in the lung
Macroscopically, the OA group showed severe and diffuse congestion (Figure 4). Microscopic analysis revealed intense capillary congestion, accompanied by multiple foci of alveolar oedema, intra-alveolar proteinaceous exudates, areas of hemorrhage, and neutrophilic inflammatory infiltrate in both the interstitium and the alveolar septa. Notably, foci of coagulation necrosis were present in the alveolar septa related to the mentioned areas of injury. At certain points, the process was even more pronounced and revealed alveolar collapse and deposits of the hyaline membrane. In the MB/OA and OA/MB groups, the changes were similar but comparatively smaller. The crucial difference was the reduction in the areas of edema, exudation, and hemorrhage. However, septal necrosis was still detected and was even more readily visualised. The Sham and MB groups showed no substantial changes (Figures 5,6).
Discussion
In ALI, the integrity of the vascular endothelial barrier is compromised; this leads to increased vascular permeability and an influx of protein-rich fluid into alveolar spaces. This study was conducted to assess the effects produced by MB in the lung tissue—particularly the effect on capillary permeability—through the inhibition of GCs in mice with OA-induced ALI.
The OA model was chosen because it replicates the essential features of ALI: OA causes irregular early inflammatory lung injury and potentially reversible changes in the permeability of leaf gas exchange and lung mechanics. One of the main advantages of this model is its reproducibility. OA administration at the same dose through the same route in distinct animals results in reasonably reproducible lung injury (6-12).
In animal models, lung injury exhibits pathological features similar to those of ALI/acute respiratory distress syndrome (ARDS), although the etiology might differ from that in humans. The OA model has thus been widely used to study the consequences of lung injury and evaluate new treatment strategies. However, because of the difference in etiology, the results must be cautiously extrapolated to ALI/ARDS in humans (13).
In addition to histological analysis, two other commonly employed methods were used in this study: measurement of WW/DW values and determination of the relative protein concentration in BAL, which are widely used as indicators of ALI (14,15). Although a simple method, the measurement of WW/DW—according to Tian et al. (16) yields values that reflect the integrity of the lung alveolar epithelium that is destroyed in OA injury (14,15). In this study, we observed increases in relative WW/DW and protein concentration in BAL in response to OA, which confirmed the loss of integrity of the alveolar-capillary membrane.
In studies on ALI in ruminants, MB attenuated, by 50%, the oedema and pulmonary hypertension induced by endotoxin administration. However, in these experiments, the beneficial effects of MB were evident only at an early stage and were exhausted after 2 h, and a parallel increase in pulmonary hypertension was observed (11,17). Leeman et al. (18) reported that MB administration resulted in increased pulmonary vascular tone, improved gas exchange, and diminution of OA-induced edema. Data obtained in this study corroborate these findings. MB effectively prevented ALI by preserving pulmonary capillary permeability. This effect was most clearly observed in the case of early MB administration: the MB pretreatment group showed a marked reduction in the total protein in BAL. These findings run counter to studies in sheep, where the early infusion of MB attenuated lung injury by reducing oedema, permeability, and pulmonary capillary wedge pressure (9,17).
Although favourable trends have been observed for permeability improvement parameters (WW/WD and protein), contrary to that seen in other experimental models, the results were not statistically significant. However, histological analysis of lung tissue showed reduced lesion areas in both pre- and post-treatment groups. In conclusion, the data collected using this experimental model were favourable only through macroscopic and histological analysis. These observations are valid for both MB infusions before or after induction of ALI, and it is possible that this difference is related to the experimental model (the OA doses, the MB doses, exposure time).
Studies on endotoxin-induced septic shock in sheep showed that cGMP and plasma NOx levels decrease following treatment with MB. Thus, MB increases systemic vascular resistance and decreases capillary permeability in conjunction with reducing pulmonary edema and morphological signs of ALI. Evgenov et al. (10) reported that MB can restore the pumping of lung lymph, probably due to the reduction of cGMP in the lymphatics. Moreover, MB treatment can temporarily restore the mean blood pressure and improve myocardial function (9). An in vitro study conducted on rat aorta reported a complete restoration of vascular hyporeactivity following the inhibition of GCs and the consequent reduction of cGMP levels (19).
NO can regulate vascular tone and thereby adjust the flow of blood to meet the metabolic demand of tissues. The production of NO is increased in the presence of inflammation. A study in dogs reported a substantial increase in both plasma and tissue NO following OA-induced ALI (20). In this study, tissue NOx values were higher for the lesion groups than for controls, but the difference was not statistically significant. Moreover, the absolute values obtained for plasma NOx were highly similar, and once again the difference was not statistically significant. One possibility is that this increase would be comparatively larger at 6 h after injury, as reported by Lai et al. (20); here, lung tissue was collected at 4 h after injury.
In studies conducted on sheep and humans with endotoxemia septic shock, circulating NOx plasma level was shown to be increased 2–7 times relative to that in healthy controls. However, Evgenov et al. (17) reported a 40% increase in plasma NOx in endotoxemic sheep, which probably reflects a modest activation of inducible nitric oxide synthase (iNOS). Plasma NOx was found to peak at 18 h after endotoxin inhalation or infusion.
Ballard-Croft et al. (21) reported that OA-induced ALI is not an appropriate model for studying inflammatory pathophysiology because the inflammation might not be required for causing lung injury. iNOS is expressed in response to proinflammatory stimuli in various cells, and this results in increased NO synthesis and the accumulation of large amounts of NO in the medium by 6 h after exposure to inflammatory agents (9,22). In this study, samples were collected at 4 h after exposure to OA, which might be one reason why an increase in NOx was not observed.
The reduction in plasma NOx detected in animals with ALI after MB treatment could indicate the inhibition of NOS. However, NOx plasma measurements might not accurately reflect the changes at the site of NO production and metabolism. Thus, tissue NOx was measured here, and the results showed that tissue NOx was lower in the MB-treatment groups as compared to that in the OA-injury group, although the difference was not statistically significant.
Fernandes et al. (22) suggested that a potential “window of opportunity” exists for the effectiveness of MB. This concept was established experimentally in rats by using a model of sepsis, which revealed three distinct 8-h windows of GC activity: in the first 8 h, NOS levels are increased and GC is upregulated; over the next 8 h, GC is not expressed and NOS is downregulated; and in the third window, NOS and GC expression is once again increased. These data highlight two points of practical and educational value: (I) MB treatment must be used based on considering the window of opportunity; and (II) this window in humans must be identified, perhaps by selecting cGMP as a biomarker (23-25).
MB exerts time-dependent effects and must be used early (in the first window), because GC expression is low in the second window, and in the third window, MB produces a late improvement of haemodynamic conditions, but under a severe metabolic scenario. These data suggest that a “window of opportunity” also exists in the case of MB therapy for pulmonary circulation.
Another possible approach is to analyse specifically iNOS; this analysis was not performed here, but it might yield detailed information regarding changes in plasma and tissue NO and the response obtained following MB treatment. Yeh et al. (26,27) used real-time PCR and western blotting to analyse the mRNA and protein expression of plasma iNOS in ALI after ischemia/reperfusion and OA treatment and observed increase in the expression of both iNOS mRNA and protein after 6 h. Certain findings support the use of MB as a potential therapeutic agent in ALI because it reduces iNOS activity (28,29).
Macroscopic examination revealed that the lungs of the mice that were not treated with MB showed increased impairment (hepatisation, edema), which was confirmed by histological examination. These data corroborate the macroscopic descriptions of Miyazawa et al. (30), who observed that following OA-induced ALI, lungs were 2–3 times heavier than normal and swollen, and that areas of the lungs were affected by haemorrhage; Miyazawa and coworkers further reported the presence of moderate to large amounts of blood foam in the trachea and the main bronchi, which was also observed in this study.
Derks and Jacobovitz-Derks (31) described in detail the pulmonary morphological alterations observed after the administration of OA in dogs; at 6–12 h after OA injection, the lungs showed alveolar flooding, capillary congestion, haemorrhage, and necrosis of the septum. In another study conducted in pigs, after 5 h of OA infusion, the same characteristics were observed (32). Similarly, the findings of this study revealed the same histological changes in rats, which corroborates other studies that presented histopathological evidence of lung injury (7,33). It is important to emphasise that the histological study was carried out randomly and blind by an expert pathologist.
The microscopy data obtained here also suggest that the main effect of MB is related to reducing congestion, intra-alveolar oedema, protein extravasation (intra-alveolar proteinaceous exudation indicating capillary injury), and hemorrhagic areas. The reduced permeability was more evident when MB was infused early rather than late. Similarly, Greca et al. (34) observed a reduction of interstitial oedema and a diminished level of lung injury coupled with decreased neutrophil infiltration in the histological analysis after early administration of MB. Tian et al. (16) observed pulmonary changes such as oedema, neutrophil infiltration, haemorrhage, diffuse alveolar collapse, and alveolar septal thickening in rats with paraquat-induced ALI. The MB treatment was started 2 h after injury, and after 24 h, the observed changes were markedly reduced. However, the histological data of this study show that MB treatment cannot prevent the development of areas of septal necrosis. Thus, the effect of MB might be limited because of the change in capillary permeability not being able to affect other mechanisms of injury induced by OA, which could include the toxic effect and the direct action of OA in alveolar and endothelial cells (13,35).
In conclusion, the data collected using this experimental model were favourable only through macroscopic and histological analysis. These observations are valid for both infusion MB, before or after induction of ALI. As previously mentioned, although favourable trends improvements for permeability parameters (WW/WD and protein) have been observed, contrary to that seen in other models, the present results were not statistically significant. It is possible that this difference is related to the experimental model (dose of OA, the MB dose, exposure time).
Acknowledgements
Partial financial sources from Sao Paulo Research Foundation (FAPESP), National Council of Scientific and Technological Development (CNPq), and Coordination of Improvement of Higher Academic Staff (CAPES).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Toya SP, Malik AB. Role of endothelial injury in disease mechanisms and contribution of progenitor cells in mediating endothelial repair. Immunobiology 2012;217:569-80. [PubMed]
- Duluc L, Wojciak-Stothard B. Rho GTPases in the regulation of pulmonary vascular barrier function. Cell Tissue Res 2014;355:675-85. [PubMed]
- Abd-Allah SH, Shalaby SM, Abd-Elbary E, et al. Human peripheral blood CD34+ cells attenuate oleic acid-induced acute lung injury in rats. Cytotherapy 2015;17:443-53. [PubMed]
- Huang B, Wang DX, Deng W. Protective effects of dexamethasone on early acute lung injury induced by oleic acid in rats. Int J Clin Exp Med 2014;7:4698-709. [PubMed]
- Liu AJ, Ling F, Li ZQ, et al. Effect of oleic acid-induced acute lung injury and conventional mechanical ventilation on renal function in piglets. Chin Med J (Engl) 2013;126:2530-5. [PubMed]
- Akella A, Sharma P, Pandey R, et al. Characterization of oleic acid-induced acute respiratory distress syndrome model in rat. Indian J Exp Biol 2014;52:712-9. [PubMed]
- Akella A, Tiwari AK, Patne SC, et al. Mesobuthus tamulus venom induces acute respiratory distress syndrome in rats involving additional mechanisms as compared to oleic acid model. Toxicon 2015;97:15-22. [PubMed]
- Crocker GH, Jones JH. Effects of oleic acid-induced lung injury on oxygen transport and aerobic capacity. Respir Physiol Neurobiol 2014;196:43-9. [PubMed]
- Evgenov OV, Sager G, Bjertnaes LJ. Methylene blue reduces lung fluid filtration during the early phase of endotoxemia in awake sheep. Crit Care Med 2001;29:374-9. [PubMed]
- Evgenov OV, Evgenov NV, Mollnes TE, et al. Methylene blue reduces pulmonary oedema and cyclo-oxygenase products in endotoxaemic sheep. Eur Respir J 2002;20:957-64. [PubMed]
- Kirov MY, Evgenov OV, Bjertnaes LJ. Combination of intravenously infused methylene blue and inhaled nitric oxide ameliorates endotoxin-induced lung injury in awake sheep. Crit Care Med 2003;31:179-86. [PubMed]
- Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008;295:L379-99. [PubMed]
- Wang HM, Bodenstein M, Markstaller K. Overview of the pathology of three widely used animal models of acute lung injury. Eur Surg Res 2008;40:305-16. [PubMed]
- Chen HI, Hsieh NK, Kao SJ, et al. Protective effects of propofol on acute lung injury induced by oleic acid in conscious rats. Crit Care Med 2008;36:1214-21. [PubMed]
- Guo Q, Jin J, Yuan JX, et al. VEGF, Bcl-2 and Bad regulated by angiopoietin-1 in oleic acid induced acute lung injury. Biochem Biophys Res Commun 2011;413:630-6. [PubMed]
- Tian ZG, Ji Y, Yan WJ, et al. Methylene blue protects against paraquat-induced acute lung injury in rats. Int Immunopharmacol 2013;17:309-13. [PubMed]
- Evgenov OV, Sveinbjørnsson B, Bjertnaes LJ. Continuously infused methylene blue modulates the early cardiopulmonary response to endotoxin in awake sheep. Acta Anaesthesiol Scand 2001;45:1246-54. [PubMed]
- Leeman M, De Beyl VZ, Gilbert E, et al. Is nitric oxide released in oleic acid lung injury? J Appl Physiol (1985) 1993;74:650-4. [PubMed]
- Wu CC, Chen SJ, Yen MH. Nitric oxide-independent activation of soluble guanylyl cyclase contributes to endotoxin shock in rats. Am J Physiol 1998;275:H1148-57. [PubMed]
- Lai TS, Cai SX, Guo ZH. Serum and lung endothelin-1 increased in a canine model of ventilator-induced lung injury. Chin Med J (Engl) 2010;123:1021-7. [PubMed]
- Ballard-Croft C, Wang D, Sumpter LR, et al. Large-animal models of acute respiratory distress syndrome. Ann Thorac Surg 2012;93:1331-9. [PubMed]
- Fernandes D, Sordi R, Pacheco LK, et al. Late, but not early, inhibition of soluble guanylate cyclase decreases mortality in a rat sepsis model. J Pharmacol Exp Ther 2009;328:991-9. [PubMed]
- Evora PR, Viaro F. The guanylyl cyclase inhibition by MB as vasoplegic circulatory shock therapeutical target. Curr Drug Targets 2006;7:1195-204. [PubMed]
- Evora PR, Rodrigues AJ, Vicente WV, et al. Is the cyclic GMP system underestimated by intensive care and emergency teams? Med Hypotheses 2007;69:564-7. [PubMed]
- Viaro F, Baldo CF, Capellini VK, et al. Plasma nitrate/nitrite (NOx) is not a useful biomarker to predict inherent cardiopulmonary bypass inflammatory response. J Card Surg 2008;23:336-8. [PubMed]
- Yeh DY, Lin HI, Feng NH, et al. Matrix metalloprotease expressions in both reperfusion lung injury and oleic acid lung injury models and the protective effects of ilomastat. Transplant Proc 2009;41:1508-11. [PubMed]
- Yeh DY, Feng NH, Chen CF, et al. Inducible nitric oxide synthase expressions in different lung injury models and the protective effect of aminoguanidine. Transplant Proc 2008;40:2178-81. [PubMed]
- Kanter M, Sahin SH, Basaran UN, et al. The effect of methylene blue treatment on aspiration pneumonia. J Surg Res 2015;193:909-19. [PubMed]
- Ayvaz S, Aksu B, Karaca T, et al. Effects of methylene blue in acute lung injury induced by blunt chest trauma. Hippokratia 2014;18:50-6. [PubMed]
- Miyazawa T, Nakagawa H, Hiramoto M, et al. Ultrastructural study on the alveolar-capillary injury with pulmonary edema induced by oleic acid in dogs. Hiroshima J Med Sci 1981;30:183-90. [PubMed]
- Derks CM, Jacobovitz-Derks D. Embolic pneumopathy induced by oleic acid. A systematic morphologic study. Am J Pathol 1977;87:143-58. [PubMed]
- Wang HM, Bodenstein M, Duenges B, et al. Ventilator-associated lung injury superposed to oleic acid infusion or surfactant depletion: histopathological characteristics of two porcine models of acute lung injury. Eur Surg Res 2010;45:121-33. [PubMed]
- Pfeifer R, Andruszkow JH, Busch D, et al. Development of a standardized trauma-related lung injury model. J Surg Res 2015;196:388-94. [PubMed]
- Greca FH, Gonçalves NM, Souza Filho ZA, et al. The role of the methylene blue as a lung protector after intestinal ischemia and reperfusion. Acta Cir Bras 2004;19:431-40.
- Peltier LF. Fat embolism. III. The toxic properties of neutral fat and free fatty acids. Surgery 1956;40:665-70. [PubMed]


