Immunological interactions in radiotherapy—opening a new window of opportunity
Introduction
After the seminal paper from Hanahan and Weinberg, the hallmarks of cancer has been defined as six biological capabilities acquired during the multistep development of cancer. Since then these hallmarks are considered to be the basic principles for understanding genesis and progression of neoplastic disease. They include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis (1). With the advancement in research after this paper they have identified some more factors which are important to the cause and updated the list in 2011 with new hallmarks, one among them is the role of immune system (2).
The major functions of immune system with regards to neoplastic process are: elimination of viruses’ that drive neoplastic transformation, resolution of acute inflammatory environment identification and elimination of transformed neoplastic cells. Tumors may evolve through a Darwinian-selection mechanism to circumvent the immune response, may induce local immune-suppression or a combination of both which results in tolerance by immune system. To use this as a therapeutic strategy we must break this tolerance that too carefully without eliciting an auto immune response.
Basic immunology
Immunity is the basic defense of the body against the foreign. Underlying genomic instabilities in cancer cells make them a foreign entity rather than one’s own normal cells. Understanding the basic concepts of immunology is essential in cancer immunotherapy. Broadly immunity is classified into innate and adaptive immunity with an extensive cross talk between them (3).
Innate immunity
It is the basic defense mechanism in the body and is an indispensible for normal immunity. It constitutes both cellular and acellular components which has a direct effect on the pathogens. Key players in the innate immune response are the basophils, eosinophils, mastcells, neutrophils, monocytes and macrophages. These constitute the cellular part and lactoferin, transferrin, interferons, TNF-α and lysozyme constitute the acellular part. Characteristic of innate immune response is the lack of memory (4); they will produce same response in each and every time when they encounter an antigen. The other key concept is that it is nonspecific. Even though it can differentiate between self and non-self, it cannot differentiate within the pathogens (e.g., Herpes virus from HPV). The innate arm of immunity recognize the self from non self through identification of cellular expression like pathogen associated molecular patterns (PAMPS) which are highly evolutionary conserved sequences. Toll like receptor family are one of the PAMPS and these cells are the primary sensors of pathogens. As a result of the activation of the innate immune system pathogens are either killed or are broken into peptides which help to activate the adaptive arm of immune system.
Natural killer (NK) cells
NK cells (3) are also phagocytes and have the ability to kill the cells directly. These are activated when a cell is not expressing class I major histocompatibility complex (MHC). Class I MHC is expressed in virtually all human cells however when there is a viral infection or carcinogenesis occurs which cause the down regulation of class I MHC so that cell is invisible to the immune system. Class I MHC is like a window into the cells which allows the immune cells to look inside for the viruses, mutated protein and helps in eradication of these cells (5-7). In this setting comes the importance of NK cells, it will be activated and kill the cells which are not expressing the class I MHC.
Adaptive immunity
It comprises mainly of T and B cells. Unlike the innate arm the components of adaptive immunity are activated by sequence specific peptides. When a B cell is activated and transformed to a plasma cell, it becomes a factory of antibodies. Those antibodies can directly kill the cells, activate the compliment mediated death, and it’s binding to antigen results in opsonisation which leads to enhanced phagocytosis of the antigen by macrophages and neutrophils. This way adaptive and innate immunity complement each other.
T cells
Helper T cells produce cytokines for the activation of B cells and cytotoxic T cells.
T regulatory cells down regulate the function of cytotoxic T cells. Their function is to control the cytotoxic cells after its finishes action on pathogens. Once the pathogen is controlled cytotoxic T cells should be regulated otherwise it will result in chronic inflammation and leads to neoplastic transformation. The key concept concept of the adaptive immunity is the presence of memory which always leads to an exaggerated immune response when there is repeated exposure (4).
Adaptive immune response only recognizes a short sequence of peptides. That peptide has to be bound in the context of class I or class II MHC. Innate cells are the main antigen presenting cells. Class I MHC is expressed in almost all nucleated cells but class II MHC is only expressed in professional antigen presenting cells like dendritic cells and macrophages. Within the MHC there is peptide binding grooves which accommodate peptides. Triggering of the T-cell-receptor complex not only requires the antigen to be recognized on the surface of an antigen-presenting cell, but also needs a second signal to be sent in a coordinated fashion through a co-stimulatory receptor. The overall effectiveness of the interaction between the MHC, T cell receptors and the signals from the co-stimulatory molecules determines the activation process (8).
Cancer immunology
Immunotherapy has now become an important part of cancer therapy, with consistent and long lasting responses being reported for a wide range of human cancers and with the advantage of a minimal toxicity profile compared to conventional cytotoxic therapies. Cancer is characterized by accumulation of altered genetic events. These events result in the expression of neoantigens, differentiation antigens or cancer testis antigens, which results in presentation of these antigenic peptides bound to (MHC-I) molecules on the surface of cancer cells. This helps CD8+ T cells to distinguish them from normal cells.
However, even when T cell responses occur, they neither provide protective immunity to the host nor could they be used as basis for therapy. To understand these we need to look into the cancer immunity cycle (9).
Immunoediting (10)
One of the important aspects of tumor is that it develops in an immunocompetent host. It means tumors have evolved through the effects of immune system. Immune system in competent host acts as both host protecting and tumor sculpting on a developing tumor. This action of immune system on developing tumor is called as tumor immunoediting. Essentially there are three steps in tumor immune editing such as elimination, equilibrium, and escape.
Elimination
It is the earliest step of immunoediting. In this step the immune surveillance leads to removal of majority of the neoplastic cells. As complete neoplastic elimination takes place no tumor cell is going to survive but the process of immune surveillance causes Darwinian selection pressure which results in escape of some cells from immune attack. This selection pressure will result in appearance of newer and newer mutations to escape an immune attack so that the antigenicity is very low.
Equilibrium
It is the longest step in immune editing. In this step the host immune system and the neoplastic cells which escape the immune cell kill reach in equilibrium. Altered genetic events as a result of the Darwinian selection pressure will produce proteins that are least immunogenic. There will be equilibrium between the immunogenicity and the altered genetic events.
Escape
In this step the equilibrium is broken in favor of neoplastic cells and best genetic alteration which can survive the immune surveillance will flourish. If it gets unchecked by therapy will result in death of the host.
Cancer immunity cycle
For an effective cell killing from anticancer immune response a series of events in a systematic order should happen in the body. These events constitute the cancer immune cycle (Figure 1). First step in the cycle is capturing of neo antigens for processing by the dendritic cells. Next step is the presentation of this antigen by the dendritic cells on MHC I or MHC II to the T cells. Along with this the signals from costimulatory molecules lead to the activation of T cells. Effectors T cell responses are generated against the cancer-specific antigens that are identified as foreign antigens. This step is actively regulated by the balance between the T regulatory cells and the effector T cell response. Activated T cells migrate to the tumor and infiltrate into the tumor bed there they identify the tumor cells which have antigens similar to the presented one and result in tumor cell kill. Killing of the cancer cell releases additional tumor-associated antigens (first step) and the cycle continues (9).
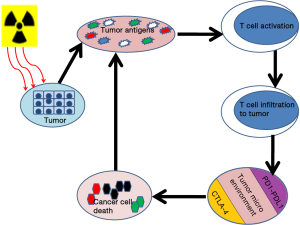
In most of the cancer cells this cycle is not well coordinated and there is always some kind of negative regulation in each step of the cycle, e.g., tumor antigens may not be detected, dendritic cells and T cells may treat antigens as self rather than foreign, T cells may not properly migrate to tumors or inhibited from infiltrating the tumor, factors in the tumor microenvironment suppress those effector cells that are produced (9).
The ultimate aim for all the cancer therapy making use of immunology is to initiate and reinitiate and propagate and amplify this cycle and in a fashion which does not initiate an auto immune response. Till now there are several interventions aimed to improve cancer immune cycle in its most optimum way, some of which are described below.
Tumor vaccination
Effort to increase the cancer control using immunization is at targeting the first step in the cancer immunity cycle. Vaccination is an attempt to activate cancer antigen-specific T cells, as well as stimulate the proliferation of these cells. But there is uncertainties concerning the identities of antigens to use, their mode of delivery, the types of adjuvants required. Presence of the negative regulators in the tumor microenvironment (represent the final steps of cancer immunity cycle) may decrease or disable antitumor immune responses before clinically relevant tumor kill occurs. As long as there is negative regulators which are acting later in the cancer immunity cycle the prospect of cancer vaccination is limited.
Adoptive T cell therapy (11)
This is one of the exciting developments in the field of immunotherapy in which autologous T cells which are activated against tumor antigens are re-infused into the patients. This had showed substantial clinical benefit in some of the hematological malignancies.
So a variety of approaches are in place to make use of once own immunity to clear the malignancy but none of them will offer a substantial benefit unless targeting the complete cancer immunity cycle.
Radiotherapy (RT)
Therapeutic vaccination is not the only approach by which we can introduce the cancer associated neo antigens. Other approaches are RT and chemotherapy which make use of the tumor that is already present in the system to generate an endogenous release of antigens. Since there is more systemic effect and less local cell kills per cycle of chemotherapy, RT may be more effective for liberating tumor associated antigens. Tumor itself represents a type of endogenous vaccine (9). The cell kill due to RT delivers immense amount of tumor antigens in various form and size to the system. This can act as tumor antigens thus avoiding the need for an exogenous delivery of antigens. But this approach is not fully overcoming the limitation of vaccination as it also acts proximal to the regulators in tumor micro environment.
Mechanism of radiation cell death
It is very interesting to note that RT is used both as an immunosuppressive agent and an immune stimulant in treatment of cancer. RT is considered as immunosuppressive in total body irradiation in conditioning regime of bone marrow transplant (12,13) and immune stimulant in most of the other solid tumors (14,15). Traditionally DNA double strand breaks were thought to be the sole mechanisms in radiation induced cell kill which results in tumor eradication and alter the tumor microenvironment through the apoptosis and mitotic cell death. Apart from this, cell kill due to RT has multi-dimensional effect on tumor survival but this may not be observed clinically due to the evasion and immune tolerance of tumor cells (which are distal steps in cancer immune cycle). Radiation damage to tumors results in the exposure of a large amount of tumor antigens, in the form of necrotic and apoptotic tumor cells and cellular debris to the immune system. The increased availability of released tumor-associated antigens for uptake by circulating dendritic cells and other antigen-presenting cells can result in tumor-specific immune attack (16,17).
RT also creates an inflammatory milieu by inducing the expression of several proinflammatory cytokines, including IL-1β and TNF-α. Increased expression of these cytokines has been linked to tumor regression, growth inhibition, and tumor-cell death (16,17). Furthermore, upregulation of MHC, costimulatory molecules, adhesion molecules, death receptors in tumor cells, surrounding stroma and vascular endothelium can also potentiate CD8+ T cell cytotoxic cell responses.
Similarly radiation induced cell damage results in increased expression of VCAM 1 on tumor cells which leads to increased migration of T cells to the tumor, translocation of calreticulin to the cell surface and the release of high-mobility group box 1 (HMGB1) by dying tumor cells, which can activate DCs through Toll-like receptor.
Traditionally RT is delivered in 1.8–2 Gy per fractions. The fractionation has impact on the immunological effects and there is evidence from animal models that changing fractionation, more favorably hypo fractionation (18) alone results in generating robust CD8+ T cell-dependent immunity. It leads to tumor reduction, reduced relapse of primary tumor, and eradication of metastasis in some settings. Potential role of RT in this setting is untapped due to the normal tissue complications. But if we can overcome this limitation by other modes this can be a game changing strategy. Unfortunately many a time the above said effect is minimal in clinical setting as the tumor will be able to evade this immune response either by immune tolerance or by immune suppression of the host. By enhancing the frequency, magnitude, and character of the immune responses induced by RT with immune modulatory agents, cancer patients could experience further improved outcomes that is targeting the distal part of the cancer immune cycle.
Therapeutic efficacy of RT has been considered so far to be solely dependent on its capacity to induce tumor cell death either on the cancer cells themselves or on the tumor stromal and vascular microenvironment. Because of this thought process developments in RT was turning around in improving technological advances in delivery, efforts to deliver higher dose, and altering dose fractionation schedule. However the efficacy can be improved if we consider whole diseased individual as a system rather than targeting tumor only and this will guide the most effective cytotoxic therapy available for localized solid tumor into a new window of opportunity (19).
There are several mechanisms in immune tolerance by cancer cells which are acting after the neo antigens, such as loss of MHC expression and up-regulation of inhibitory molecules of immune response like PD-L1, cytotoxic T lymphocyte antigen-4 (CTLA-4). Hence there are several layers of immune regulation by which tumor escape from the immunological effects.
Cytotoxic T lymphocyte antigen-4 (CTLA-4) (20,21)
It is the key regulator of T cell response and tolerance to self-antigen. This is one of the mechanism by which body can differentiate the self from non-self-environment however the intelligent tumor cells will make use of this as an opportunity to escape from the T cell mediated cell kill. Activation of T cell requires primarily two signals. First signal is from the presentation of antigenic peptides in the context of MHC, second is from binding of CD28 co-receptor to costimulatory molecules CD80 (B7-1) and CD86 (B7-2) which results in activation, T-cell proliferation and cytokine production. CTLA-4 will compete with costimulatory molecules for the coreceptors thus leads to competitive inhibition. CTLA-4 engagement regulates integrin-dependent motility and prevents T cells from forming long-term interactions with APCs or target cells, which are necessary to sustain T-cell activation and cytotoxic activity. CTLA-4 is constitutively expressed in T-Regs and promotes highly suppressive cytokine TGF-β.
So in the highly immune compromised tumor micro environment persistent tumor antigen exposure causes the exhaustion of T cells along with higher expression of CTLA-4 and other immune checkpoint receptors which contribute to a significantly reduced antitumor immune response.
Programmed death (22)
PD-1 is another important inhibitory receptor expressed by T cells. The activation of PD-1 plays an important role in maintenance of peripheral tolerance. There are two PD1 ligands which have been identified i.e., PD-L1 and PD-L2. Expression of PD-L2 is limited to myeloid cells. PD-1/PD-L1 axis is one of the determinants of modulation of T cell function. It regulates the T cell function through T cell receptor signal transduction and inducing apoptosis of activated T cells.
Interaction of radiation and immunology
Till now most of the effort in cancer treatment is by either targeting the tumor cell or targeting the immune system. Each of these modalities was independently thought to cause cure but it failed to deliver its purpose in many solid tumors. The combination effects are promising and can results in a magical cure not only in localized disease but also for the metastatic and advanced disease. Among all the negative regulators of the cancer immune cycle the tumor microenvironment is thought to be the most important. Recent advances in clinical research aim to target these negative regulators. Most important are the PD-1/PD-L1 and CTLA-4.
When there is a strong endogenous antitumor immune response, targeting the up regulated negative regulators in the microenvironment will result in enhanced tumor control. But when there is no or reduced antitumor response, targeting inhibitory molecules will be a futile effort. In that setting, agent who can induce an anti-tumor immune response will be more effective.
PD-L1-blocking therapy reinvigorates exhausted CD8+ T cells. CTLA4-blocking therapy predominantly decreases TReg cell numbers and, together, these immune checkpoint inhibitors increase the CD8/TReg ratio and promote the peripheral clonal expansion of TILs. Role of radiation is to diversify the T cell receptor repertoire of tumor infiltrating lymphocytes. It also shapes the repertoire of the expanded peripheral clones. RT and the immune targeted agents together act synergistically and elicit an immune response locally and systemically and may results in response to even non-irradiated areas. This field seems to be promising pathway in future.
Clinical application and trials in immunotherapy and RT
Although there was evidence for contribution of immune system to the therapeutic response of radiation in preclinical setting since 1970, however it is last 10 years or so when immunotherapy concurrent with RT has turned up in clinics in a big way. There are lots of trials with experimental molecules both in preclinical and clinical settings going on. Addressing all the clinical trials are beyond the scope of this review. We are focusing on few important clinical trials (Table 1).
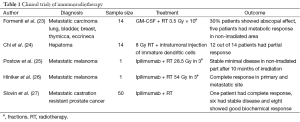
Full table
The first clinical trial combined a recombinant cancer vaccine with standard definitive RT in patients with localized prostate cancer. A randomized phase II study was conducted with patients receiving local radical RT with or without vaccine. Primary endpoint of the trial was immunologic response, with secondary endpoints of safety and clinical response. A total of 30 patients were enrolled in the study. Patients in the combination arm received a priming vaccine of recombinant vaccinia (rV) expressing prostate-specific antigen (PSA) (rV-PSA) admixed with rV expressing the co-stimulatory molecule B7-1 (rV-B7-1), followed by monthly booster vaccines with recombinant fowl pox (rF)-PSA. The vaccines were given with local granulocyte-macrophage colony-stimulating factor (GM-CSF, Leukine) and low dose systemic IL-2. There was no detectable increases in PSA-specific T cells in the RT-only arm but the 13 patients who completed the vaccination and radiation course had at least 3-fold increase (P<0.0005) PSA specific T cells. There was also evidence of de novo generation of T cells to prostate-associated antigens not present in the vaccine, a phenomenon described as “antigen cascade”, among the patients treated in the combination arm, providing indirect evidence of immune-mediated tumor-killing (28).
The New York Group designed a “proof-of principle” clinical trial, aimed at detecting an abscopal response (a response distant to the radiation field) after GM-CSF in metastatic cancer patients. Eligible subjects for this study were patients with at least three measurable lesions, who had stable or progressive disease during chemotherapy. The same chemotherapy was continued but RT was added to one lesion, at a dose of 3.5 Gy × 10 fractions over a period of 2 weeks. After 1 week of radiation, GM-CSF, 125 µg/m2, was given subcutaneously and repeated daily for 14 days. Assessment of response was performed by PET-CT. Currently 14 patients have accrued to this trial. Tumor histology was: lung cancer (6), poorly differentiated thymic carcinoma (2), breast carcinoma (4), bladder carcinoma (1), eccrine carcinoma. Twelve patients could be evaluated for response (i.e., had completed treatment and data from PET/CT before and following therapy were available): four achieved an abscopal response (30%). In five patients a decrease in standardized uptake value (SUV) of non-irradiated lesions was observed on PET scan. In three patients the response was preceded by a “flare” effect at PET (23).
After radiation exposure, the role of dying tumor cells in sensitizing dendritic cells was tested in a phase I clinical trial of fourteen patients with hepatoma (24). A single dose of 8 Gy of external-beam radiation therapy to the tumor was followed by an intra tumoral injection of immature DCs, delivered on days 2 and 24. Twelve of fourteen patients had a partial response, and most patients had increases in alpha-fetoprotein-specific immune responses by cytokine-release assay and ELISPOT.
Postow et al. (25) reported about a patient whose metastatic melanoma regressed with ipilimumab and concurrent palliative RT. The patient had received 28.5 Gy in 3 fractions to an area next to the spine. Post treatment CT scan revealed that masses elsewhere in the spleen and hilar lymph nodes had also regressed and eventually reached the point of stable minimal disease 10 months after radiation. This case prompted a pilot study by Hiniker et al. (26) to combine ipilimumab and concurrent RT for a patient with asymptomatic melanoma. That patient received a higher dose of 54 Gy in three fractions and showed a complete response in both the primary tumor and the metastatic lesions. In a phase I/II clinical study, Slovin et al. (27) used ipilimumab along with radiation in metastatic castration resistant prostate cancer. A total of 50 men were given ipilimumab (four 10 mg/kg doses) plus RT (8 Gy fractions to each lesion for 3 weeks), one patient had complete response, six had stable disease and eight showed good biochemical response.
With efficacy of CTLA4 blockers being proved in case reports or phase II trials anti-PD-1/PD-L1 mAbs have drawn much interest for their potential use in lung or colon cancer (29) and in combination with CTLA-4 blockade for melanoma.
The study by Verbrugge et al. (30) showed neither anti-PD-1 mAb nor radiation when given alone was effective in a murine model of triple-negative breast cancer. However, the addition of anti-PD-1 mAbs enhanced the curative capacity of RT and CD137 (an agonist antibody for costimulatory molecule 4-1BB) against both established tumors and secondary tumor challenge, indicating that the combined regimen conferred antitumor immune responses and memory.
Conclusions
Radiation has been a back bone of cancer therapy since the early 20th century and is implemented in around half of latest cancer treatment plans. RT was traditionally considered as a localized form of treatment. It was thought that it has no effect on distant metastasis. With the emergence of stereotactic body radiotherapy (SBRT) and its ability to treat the oligo-metastasis there was a paradigm shift from the conventional thought process. Though SBRT is used for treating oligo metastasis but it is a tumor directed therapy only. SBRT is not the tool where the exact systemic effect of radiation has been explored. Immunotherapy concurrent with RT has opened that window for radiation to treat systemic disease with localized treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer 2004;4:11-22. [PubMed]
- Delves PJ, Martin SJ, Burton DR, et al. Roitt's Essential Immunology. 12th edition. New York: Wiley Blackwell, 2011.
- Comber JD, Philip R. MHC class I antigen presentation and implications for developing a new generation of therapeutic vaccines. Ther Adv Vaccines 2014;2:77-89. [PubMed]
- Cohen GB, Gandhi RT, Davis DM, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 1999;10:661-71. [PubMed]
- Shastri N, Nagarajan N, Lind KC, et al. Monitoring peptide processing for MHC class I molecules in the endoplasmic reticulum. Curr Opin Immunol 2014;26:123-7. [PubMed]
- Lodish HF, Berk A, Zipursky SL, et al. Molecular cell biology. 4th edition. New York: WH Freeman, 2000.
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [PubMed]
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. [PubMed]
- Gattinoni L, Powell DJ Jr, Rosenberg SA, et al. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 2006;6:383-93. [PubMed]
- Rossi HA, Becker PS, Emmons RV, et al. High-dose cyclophosphamide, BCNU, and VP-16 (CBV) conditioning before allogeneic stem cell transplantation for patients with non-Hodgkin's lymphoma. Bone Marrow Transplant 2003;31:441-6. [PubMed]
- Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009;15:1628-33. [PubMed]
- Sologuren I, Rodríguez-Gallego C, Lara PC. Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects. Transl Cancer Res 2014;3:18-31.
- Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol 2012;2:191. [PubMed]
- Burnette B, Weichselbaum RR. Radiation as an immune modulator. Semin Radiat Oncol 2013;23:273-80. [PubMed]
- Kwilas AR, Donahue RN, Bernstein MB, et al. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front Oncol 2012;2:104. [PubMed]
- Burnette B, Weichselbaum RR. The immunology of ablative radiation. Semin Radiat Oncol 2015;25:40-5. [PubMed]
- Schaue D, McBride WH. Opportunities and challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol 2015;12:527-40. [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [PubMed]
- Pilones KA, Vanpouille-Box C, Demaria S. Combination of radiotherapy and immune checkpoint inhibitors. Semin Radiat Oncol 2015;25:28-33. [PubMed]
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458-68. [PubMed]
- Formenti SC, Friedman K, Chao K, et al. Abscopal response in irradiated patients: results of a proof of principle trial. Int J Radiat Oncol Biol Phys 2008;72:S6-7.
- Chi KH, Liu SJ, Li CP, et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother 2005;28:129-35. [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [PubMed]
- Hiniker SM, Chen DS, Reddy S, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol 2012;5:404-7. [PubMed]
- Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 2013;24:1813-21. [PubMed]
- Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res 2005;11:3353-62. [PubMed]
- Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 2012;18:6580-7. [PubMed]
- Verbrugge I, Hagekyriakou J, Sharp LL, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res 2012;72:3163-74. [PubMed]


