
Eosinophils and associated parameters in different types of skin diseases related to elevated eosinophil levels
Introduction
Eosinophils have been traditionally considered as beneficial against infection. The infiltration of eosinophils in inflammatory site revealed their ambiguous functions. A wide range of skin disorders are associated with eosinophil infiltration and the possibility of peripheral blood eosinophilia. Exogenous stimuli provoke innate or adaptive type 2 response, causing the group 2 innate lymphoid cells (ILC2) or T helper 2 (Th2) cells, respectively, to release type 2 cytokines such as IL-5 and IL-13. Recruited to the tissue and activated by these cytokines, eosinophils release toxic granule proteins (eosinophil peroxidase, eosinophil cationic protein, eosinophil-derived neurotoxin, and major basic protein) and various cytokines. Besides exerting functions in host defense especially against helminth, eosinophils can cause edema by producing leukotrienes, and induce blister formation and pruritus in diverse skin disorders (1,2). Chronic spontaneous urticaria has been considered closely related to mast cells, but not eosinophils, with recent study revealing activation of eosinophils in the affected lesions and improvement after treatments against IL-5 (3). Atopic dermatitis (AD) is closely associated with type 2 T helper cell immunity and elevated immunoglobulin E (IgE) levels, resulting in relapses of pruritis and eczema. Impaired epidermal function, skin microbiome dysregulation, and abnormalities of Th2 immunity interact with one another to accelerate the occurrence of AD. It is estimated that AD affected 20% of children and 10% of adults in high-income countries (4). Bullous pemphigoid (BP) is an autoimmune blistering skin disease in which the body forms antibodies against BP180 and/or BP230, which are important components of hemidesmosomes. Antibodies against BP180 and/or BP230 could ultimately result in dermal-epidermal splitting and the formation of tense blisters. It has also been shown that eosinophils play an important role in blister formation (5). A survey in France showed that the incidence of BP was 0.00217% per year (6). The onset of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, also called drug-induced hypersensitivity syndrome, is usually associated with drug use and is characterized by systematic manifestations that include rashes, fever, lymphadenopathy, eosinophilia, and liver dysfunction (7). Although the pathophysiology of DRESS remains unclear, it is widely acknowledged to result from delayed type IV hypersensitivity reactions (8). A retrospective study in the US identified the number of potential DRESS syndrome cases as 2.18 per 100,000 patients from 1980 to 2016 (9). With an absolute elevated peripheral eosinophil count ≥1,500/µL and a related wide array of clinical manifestations, hypereosinophilic syndrome (HES) is a group of various diseases without specific pathogenesis (10,11). Although no exact data are available, the prevalence of HES is estimated to be between 0.000315% and 0.0063% (12).
Although all of the abovementioned skin disorders seem to present with eosinophilia, the importance of eosinophils in each disease remains unknown. There is a need for better parameters to use during the course of diagnosis, treatment, or follow-up. In this cross-sectional study, peripheral blood samples from Chinese patients with AD, BP, DRESS, and HES and healthy controls were collected. The white blood cell (WBC) count, peripheral eosinophil count, peripheral basophil/WBC percentage, and IgE level were compared and described. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-99/rc).
Methods
Study design and data source
In this cross-sectional study conducted from 1 January 2018 to 30 September 2020, blood test results, including the WBC count, peripheral eosinophil count, peripheral basophil/WBC percentage, and IgE level, were collected from patients diagnosed with the 4 diseases of AD, BP, DRESS, and HES (Table 1). The AD patients were diagnosed according to the criteria of Hanifin (13), and the BP patients were diagnosed by pathological biopsy. When the peripheral eosinophil count exceeded 1,500 and other diseases were excluded, patients were considered to have HES (14). The DRESS patients were selected based on the Japanese Consensus Group diagnostic criteria (15). The healthy controls comprised 621 randomly selected volunteers and were compared with 115 AD patients, 75 BP patients, 55 DRESS patients, and 119 HES patients. The sample size was decided by the number of cases in the area.
Table 1
| Parameters | AD | BP | DRESS | HES | Control | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25th | MD | 75th | 25th | MD | 75th | 25th | MD | 75th | 25th | MD | 75th | 25th | MD | 75th | ||||||
| Age | 20 | 25 | 37 | 57 | 68 | 76 | 35 | 51 | 68 | 57 | 65 | 71 | 29 | 32 | 38 | |||||
| WBC (×109/L) | 6.19 | 7.45 | 9.14 | 6.90 | 8.20 | 11.19 | 6.95 | 10.49 | 14.56 | 7.03 | 8.32 | 10.43 | 4.95 | 5.89 | 6.91 | |||||
| EOS (×106/L) | 374 | 748 | 1166 | 22 | 154 | 902 | 66 | 352 | 1122 | 880 | 1573 | 2387 | 71 | 112 | 192 | |||||
| EOS% (%) | 4.9 | 10.71 | 15.98 | 0.27 | 2.02 | 13.74 | 0.86 | 3.91 | 10.14 | 12.71 | 19.83 | 28.39 | 1.30 | 1.90 | 3.30 | |||||
| BASO (×106/L) | 1.850 | 3.020 | 5.200 | 0.800 | 2.150 | 3.300 | 1.170 | 2.695 | 4.215 | 0.000 | 2.845 | 5.902 | 19.270 | 28.600 | 39.905 | |||||
| BASO% (%) | 0.20 | 0.40 | 0.70 | 0.10 | 0.20 | 0.40 | 0.10 | 0.30 | 0.48 | 0.00 | 0.30 | 0.78 | 0.30 | 0.50 | 0.70 | |||||
| IgE (IU/mL) | 1276.8 | 2640.0 | 2856.0 | 470.4 | 1668.0 | 2784.0 | 45.8 | 307.2 | 999.6 | 233.8 | 643.2 | 2640.0 | 44.4 | 115.0 | 320.4 | |||||
AD, atopic dermatitis; BP, bullous pemphigoid; DRESS, drug reaction with eosinophilia and systemic symptoms; HES, hypereosinophilic syndrome; WBC, peripheral white blood cell count; EOS, peripheral eosinophil count; EOS%, peripheral eosinophil/WBC percentage; BASO, peripheral basophil count; BASO%, peripheral basophil/WBC percentage; MD, median values; 25th, 25th percentiles; 75th, 75th percentiles.
The included patients demonstrated relatively severe symptoms and were admitted to Huashan Hospital. Most of the participants were treated with steroids, while those who showed little improvement began taking compound glycyrrhizin tablets. After 90% of the affected skin area had improved and the pruritis score was reduced to below 1, most of the participants were discharged. Data were collected at admission, 1 week after discharge, and 2 weeks after discharge.
Statistical analysis
Data were analyzed using the software SPSS 26.0 (IBM Corp., Chicago, IL, USA). In the Shapiro-Wilk test of normality, the null hypothesis was rejected for all parameters, divided by different diseases, when α=0.1 and could not be reverted by normality transformation. Therefore, all of the comparisons among diseases were performed by nonparametric Kruskal-Wallis tests with α=0.05. Missing data were excluded through pairwise deletion of cases.
The Spearman correlation coefficients were calculated for IgE eosinophils, IgE basophils, and eosinophil basophils. The distribution of ages and genders was described for different disease groups.
Ethical statement
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Huashan Institutional Review Board, Fudan University (KY 2020-1135) and informed consent was taken from all the patients.
Results
Descriptive analysis by age and gender
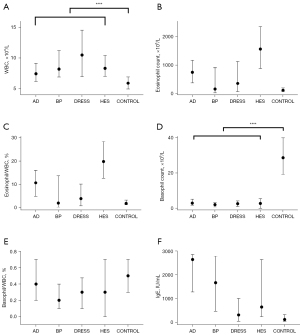
Except for BP–HES, all other pairwise comparisons demonstrated significant differences in age (shown in Figure 1A). All of the figures in this article were created by Prism 8 (GraphPad Software Inc., La Jolla, CA, USA).

The majority of our healthy controls were young people. To test the possible interference caused by the age of the controls, the data were divided by age into 5 groups from 0 to 100, with each group representing a span of 20 years. There was no difference in the distribution of age groups within each group, indicating that age had little impact on the results of this study.
Pearson’s chi-square test showed that only the HES group had a significantly larger proportion (α=0.05) of male participants (Figure 1B).
Parameters in different groups
WBC
The control group had a significantly lower peripheral WBC count than the other groups (P values all equal to 0), with no significant difference among the 4 disease groups (Figure 2A).
Eosinophils
Both the peripheral eosinophil count and eosinophil/WBC ratio were compared among the groups. These 2 parameters showed almost the same tendency among groups, with slight differences. The control group had the lowest eosinophil count and eosinophil/WBC ratio, similar to the BP group. Participants with DRESS had significantly higher levels than the control group and similar levels to the BP group. In contrast, the HES and AD groups had significantly higher levels than the other 3 groups (Figure 2B,2C).
Basophils
Both the peripheral basophil count and the basophil/WBC ratio were calculated. The control group had a significantly higher basophil count than the other groups, with no significant difference among the 4 disease groups (Figure 2D).
Although the 4 disease groups seemed similar in terms of both peripheral WBC and basophil counts, the basophil/WBC ratios were different. Only the pairwise comparisons N–AD, AD–HES, HES–DRESS, and DRESS–BP presented no significant difference. This finding, combined with the information from the box plot, indicated that the disease groups can be ordered from highest to lowest as N–AD–HES–DRESS–BP, with only the adjacent 2 groups showing similar values (P value higher than 0.05) (Figure 2E).
IgE
The control group had significantly lower IgE levels than the other groups. Patients with DRESS had an IgE level that was statistically higher than that of the control group but lower than that of the other 3 groups. Participants with AD, BP, and HES showed significantly elevated IgE levels, while AD patients showed the most prominently elevated IgE levels (Figure 2F).
Eosinophil/basophil ratio
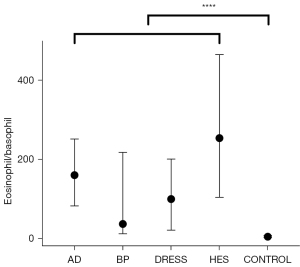
Some data were missing due to the 0 peripheral basophil count; thus, only 99 AD patients, 67 BP patients, 43 DRESS patients, 80 HES patients, and 615 healthy controls were included. The eosinophil/basophil ratio of the control group differed significantly from that of the disease groups, and the distribution was somewhat limited (Figure 3).
Correlations between parameters
The Spearman correlation coefficients of IgE–eosinophils, IgE–basophils, and eosinophils–basophils were calculated and compared. There was no correlation between IgE levels and basophils (α=0.05) in any group. Eosinophils and IgE were weakly correlated in only the BP and control groups. Furthermore, eosinophils and basophils were weakly correlated only in the HES and control groups.
Parameters and disease severity
By comparing the data collected before admission and after discharge, the impact of treatment effects and disease severity on the parameters could be roughly described.
Basophils and IgE
The basophil/WBC ratio and IgE levels were measured only twice, once before admission and once 1 week after discharge. There were no sufficient data collected in the BP, DRESS, and control groups to support the analysis. Using the related-samples Wilcoxon signed rank test, IgE and basophil/WBC ratio of 3 samples from the AD group, IgE of 4 samples from the HES group, and the basophil/WBC ratio of 5 samples from the HES group were calculated. The IgE level and basophil/WBC ratio did not change significantly after discharge.
Eosinophils and WBCs
Eosinophils and WBCs were measured 3 times: before admission, 1 week after discharge, and 2 weeks after discharge. There were 62 samples from the AD group, 61 from the BP group, 52 from the HES group, and 15 from the DRESS group at 1 week post discharge, but at 2 weeks post discharge, there were only 10 from the AD group, 3 from the BP group, 26 from the HES group, and 8 from the DRESS group. When all of the data collected from 3 repeated measurements were considered together, both eosinophils and WBCs count did not satisfy the requirements for spherical symmetry, and the multivariate tests demonstrated no significant difference, which may be the result of the limited sample size.
Furthermore, when only data from before admission and 1 week after discharge were considered, which resulted in a larger sample size, the related-samples Wilcoxon signed rank test showed a significant decrease in the peripheral eosinophil count in the disease groups. In this analysis, 132 out of 189 participants experienced a decrease in the peripheral eosinophil count after the alleviation of the disease, regardless of disease group. Nonetheless, the discharged patients still had significantly higher eosinophil counts than the healthy controls.
Discussion
Eosinophil, basophil, WBC, and IgE levels were compared across the 4 disease groups. All patients showed increased peripheral eosinophil counts, eosinophil/WBC ratios, and IgE levels, with variance among the different disease groups. Although the decrease in the peripheral basophil count in the disease groups was not in line with the critical role of basophils, it might be explained by the fact that basophils were strongly recruited but weakly stimulated to proliferate. Eosinophils were shown to be a more useful biomarker for the treatment effect than the other parameters considered in this study given their significant decrease after discharge regardless of the disease group.
The distribution of age and gender was also analyzed. The AD patients tended to be younger, the BP and HES patients tended to be older, and the DRESS patients were relatively evenly distributed. Only the HES group showed a predominance of males, which is consistent with previous studies (16).
Function of eosinophils
Our data indicated that patients with AD, HES, and DRESS had obviously increased levels of peripheral eosinophils, a finding that is consistent with previous studies illustrating the role of eosinophils in the pruritus and itching associated with various skin diseases (17). By releasing toxic granule proteins and producing leukotrienes, either directly regulating blood vessels or indirectly stimulating mast cells, eosinophils also contribute to tissue edema (2).
Among these skin diseases linked to increased eosinophil levels, the AD and HES groups had the highest levels of peripheral eosinophils, indicating that eosinophils have an important role in the pathogenesis of these diseases. As previously reported, a study using the MC903 mouse model showed that depletion of eosinophils obviously improved impaired skin barrier function and alleviated the symptoms of AD (18).
Eosinophils are also indispensable in the formation of blisters in BP patients. They are observed lining along dermal-epidermal junction, and locating in blisters. Although autoantibody IgE has been shown to mediate BP blister formation in a humanized IgE receptor mouse model, it fails to do so when eosinophils are deficient (19,20). Eosinophils, after activated by IL-5, could cause splitting of dermal-epidermal junction in the absence of BP antibodies (20), but clinical trial using anti-IL-5 antibody mepolizumab failed to ameliorate BP symptoms (21). However, our data showed no significant difference between the BP and control groups in terms of either peripheral eosinophil count or the eosinophil/WBC ratio. This contradictory result might be attributed to the rough balance of eosinophils proliferated and recruited out of the peripheral pool.
A previous cross-sectional study reported that BP patients with eosinophilia were significantly older than patients without eosinophilia (22), which does not concur with our data. The age distribution showed no significant difference in patients with and without eosinophilia. Ethnicity might account for this contradiction.
Peripheral eosinophilia had been a key feature of DRESS, and cutaneous eosinophil infiltration was rarely reported. Treatments against IL-5 proved effective, suggesting the elevated eosinophil level in DRESS primarily depends on IL-5 (2).
The IL-5 and IL-3 production from aberrant T cells could explain HES in part. Perivascular infiltration of eosinophils and lymphocytes could be found in those patients (2). Our results also discovered significant increased eosinophil level in HES patients.
For skin diseases associated with elevated peripheral eosinophils, anti-inflammatory approaches and therapies targeting eosinophils or related cytokines may be effective. Corticosteroids and calcineurin inhibitors such as tacrolimus could suppress the function of eosinophils as well as those cytokines stimulating them. Antibodies blocking the upstream or downstream pathways of eosinophil are also useful. Literature stated that antibodies against IL-5, CD52, IL-13, IL-31 and thymic stromal lymphopoietin could reduce eosinophil inflammation (23). Apart from these traditional therapies, new discoveries point out possible future directions. Eosinophils with defected autophagy functions are presented with increased effector functions, exaggerating tissue inflammatory (24). Therapies targeting autophagy regulatory pathways may also be promising.
Function of basophils
Basophils are also associated with pruritus (25). Evidence has demonstrated that basophils infiltrate skin lesions, especially in BP and AD (26), and that basophils may have a greater influence than effector cells given their ability to present antigens in allergen-induced T(H)2 responses in vitro and in vivo (27).
Despite the various important roles of basophils, the disease groups in this study did not demonstrate elevated peripheral basophils, which is consistent with a previous study indicating that the number of basophils is significantly elevated in the skin, but not in the blood, in AD (28). Unlike eosinophils, the involvement of basophils in these skin diseases was not accompanied by peripheral basophilia. Eosinophils and basophils may both be recruited to the affected skin area, but basophils are less strongly stimulated to proliferate in peripheral blood. Whether this theory applies to other skin diseases requires further investigations involving biopsies of skin lesions.
In AD patients, basophils secrete histamine and IgE when induced by antigens (29). By eliciting an interleukin-3-independent basophil response, Th2 cell-dependent immunity in AD patients could be enhanced (30). A recent study also found that FcεRIa is upregulated on basophils in AD-associated inflammation (31). These significant effects of basophils on AD might account for our finding that the AD group presented a higher basophil/WBC ratio than the DRESS and BP groups. Recent study suggests IL-37b could reduce the activation of basophils, and alleviate atopic dermatitis (32). More convincing evidence might be observed in skin biopsy results.
Although AD resembles BP in terms of both peripheral basophil and peripheral WBC counts, the AD patients had a higher basophil/WBC ratio than BP patients. This might indicate autoimmunity in BP, with higher levels of lymphocytes.
Function of IgE
By combining to the receptor FcεRI, IgE could induce the degranulation of mast cells and basophils, attracting leukocytes and amplifying the inflammatory process (33). All of the disease groups had significantly higher IgE levels than the control group. However, some healthy control samples presented elevated IgE levels, while some patients presented normal IgE levels, suggesting greater variation in IgE than in other parameters measured.
It has been reported that basophils activated via their high-affinity IgE receptor could prolong basophil-endothelial interactions, inducing the accumulation of eosinophils (34,35). The upstream role of IgE is consistent with our observation of elevated IgE levels in the disease groups.
Of the 4 disease groups, the AD group demonstrated the highest IgE level, indicating the indispensable role of IgE in AD. A study showed that AD patients treated with systemic CyA demonstrated improved symptoms along with a reduction in IgE autoreactivity (36).
The criterion of 200 U/mL IgE is usually used to discriminate between intrinsic and extrinsic AD (37). It has been reported that the classic extrinsic phenotype applies to 80% AD patients and is associated with a higher likelihood of elevated IgE levels, eosinophilia, a history of atopy, and filaggrin mutations. The other 20% of AD patients have normal IgE levels, late disease onset, preserved skin barrier function, and no history of atopy (37). In our data, only 8 patients had IgE levels lower than 200 U/mL, while the other 102 patients had IgE levels higher than 200 U/mL. No significant difference in eosinophils existed between the intrinsic and extrinsic AD patients in this study, which might be attributed to the relatively severe condition of inpatients.
As an autoimmune disease, BP also demonstrated elevated IgE levels similar to those of AD, including autoreactive IgE specific for BP180 and BP230. Skin biopsies of BP patients showed self-reactive IgE binding to mast cells and/or eosinophils (38).
Eosinophil/basophil ratio
Compared with healthy controls, all the patients showed lower peripheral basophil counts and higher eosinophil/basophil ratio. Although unable to effectively diagnose patients of certain disease, these 2 parameters could be used to exclude healthy controls. Those with decreased peripheral basophil counts and increased eosinophil/basophil ratio are more likely to have these 4 skin disorders. Combining both eosinophil and basophil, this ratio may have the potential to better describe different changes in eosinophils and basophils. Whether this parameter could be used for diagnosis requires further research.
Correlations
In the control group, weak rank correlations existed between both eosinophils–IgE and eosinophils–basophils. Similar weak rank correlations were also present between eosinophils–IgE in the BP group and between eosinophils–basophils in the HES group. The absence of this weak correlation in the other diseases indicated the discrete functions of IgE, eosinophils, and basophils. Basophil counts and IgE levels demonstrated no rank correlation in any group. As allergic reactions are always associated with the elevation of IgE, this result might provide clues about the other functions of eosinophils and basophils, in addition to effector cells, in allergic reactions.
AD, prominently resulted from type 2 inflammation, showed evidently increased IgE and eosinophil levels. IgE might contribute to eosinophil recruitment and activation through degranulation of mast cells or direct modulation of eosinophils (39). Although both eosinophil and IgE are crucial for this allergic disease, they demonstrate no correlation, indicating distinct regulatory pathways. BP, as a typical auto-immune disease, showed evidently increased IgE level, slightly increased eosinophil level, and weakly correlated eosinophils-IgE results. This might indicate that these eosinophils were majorly recruited and activated in synchronization with IgE, with little stimulation from other pathways. DRESS has been described as a drug reaction owing to type 4 hypersensitivity reaction, with slightly increased eosinophil and IgE levels, which might be secondary to the T cell functioning. For HES patients, apart from the significantly elevated eosinophil level, the IgE level showed conspicuous variation. Since the diagnosis of HES simply depends on peripheral eosinophil counts, it is likely that detailed subdivisions await discovery.
Eosinophils may act as biomarkers of treatment effects
Numerous biomarkers are available for evaluating the severity or activity of a range of skin diseases. However, to make this valuation easier and more widely applicable, our data suggest that the peripheral eosinophil count is a reflection of the treatment effects. After their discharge from the hospital, 132 patients showed significantly decreased peripheral eosinophil counts, 38 patients showed a minor increase, and 19 patients maintained the same result, regardless of the disease type. Such significant changes were observed only in eosinophils and not in other parameters. Therefore, it can be concluded that, compared with other parameters in this study, eosinophils are an effective biomarker for evaluating treatment outcomes, regardless of disease type.
According to the results of this cross-sectional study of AD, BP, DRESS, and HES patients, both eosinophils and basophils have indispensable roles in the pathogenesis of these diseases that in some cases are demonstrated only in the skin and not in the blood. Differences in the roles of eosinophils, basophils, and IgE could account for the weak correlation among these parameters and may indicate that eosinophils have more functions beyond evoking allergic reactions. Compared with other parameters in this study, eosinophils can serve as a better biomarker for determining treatment effects.
To our knowledge, this is the first study to compare eosinophil, basophil, and IgE levels across skin diseases, including AD, BP, HES, and DRESS. However, a limitation of this study is that skin biopsies were not applied. Peripheral results can only partly reveal the underlying pathogenesis and are not always parallel to biopsy results. Although some of these diseases are considered rare, the sample size could be further enlarged to avoid biases and confirm our findings. A 2-week follow-up was designed to examine the changes in results after discharge, but this duration is too short to present an accurate picture for chronic diseases. A longer follow-up might provide more information. As this study was conducted in China and only in-patients were recruited, patients of other ethnicities or with mild conditions may return different outcomes.
Acknowledgments
Funding: This work was supported by the Shanghai Municipal Health Commission for clinical research in the health industry (20194Y0126). The funding source had no involvement in this study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-99/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-99/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-99/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Huashan Institutional Review Board, Fudan University (KY 2020-1135) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leiferman KM, Peters MS. Eosinophil-Related Disease and the Skin. J Allergy Clin Immunol Pract 2018;6:1462-82.e6. [Crossref] [PubMed]
- Radonjic-Hoesli S, Bruggen MC, Feldmeyer L, et al. Eosinophils in skin diseases. Semin Immunopathol 2021;43:393-409. [Crossref] [PubMed]
- Altrichter S, Frischbutter S, Fok JS, et al. The role of eosinophils in chronic spontaneous urticaria. J Allergy Clin Immunol 2020;145:1510-6. [Crossref] [PubMed]
- Weidinger S, Novak N. Atopic dermatitis. Lancet 2016;387:1109-22. [Crossref] [PubMed]
- Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet 2019;394:882-94. [Crossref] [PubMed]
- Joly P, Baricault S, Sparsa A, et al. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol 2012;132:1998-2004. [Crossref] [PubMed]
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: A retrospective study. J Am Acad Dermatol 2020;82:606-11. [Crossref] [PubMed]
- Bellón T. Mechanisms of Severe Cutaneous Adverse Reactions: Recent Advances. Drug Saf 2019;42:973-92. [Crossref] [PubMed]
- Wolfson AR, Zhou L, Li Y, et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome Identified in the Electronic Health Record Allergy Module. J Allergy Clin Immunol Pract 2019;7:633-40. [Crossref] [PubMed]
- Klion AD, Ackerman SJ, Bochner BS. Contributions of Eosinophils to Human Health and Disease. Annu Rev Pathol 2020;15:179-209. [Crossref] [PubMed]
- Chen YY, Khoury P, Ware JM, et al. Marked and persistent eosinophilia in the absence of clinical manifestations. J Allergy Clin Immunol 2014;133:1195-202. [Crossref] [PubMed]
- Crane MM, Chang CM, Kobayashi MG, et al. Incidence of myeloproliferative hypereosinophilic syndrome in the United States and an estimate of all hypereosinophilic syndrome incidence. J Allergy Clin Immunol 2010;126:179-81. [Crossref] [PubMed]
- Jon M, Hanifin GR. Diagnostic features of atopic dermatitis. Acta Dermatovener (stockholm) Suppl 1980;92:44-7.
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol 2012;130:607-12.e9. [Crossref] [PubMed]
- Shiohara T, Iijima M, Ikezawa Z, et al. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol 2007;156:1083-4. [Crossref] [PubMed]
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in Children and Adults: A Retrospective Comparison. J Allergy Clin Immunol Pract 2016;4:941-7.e1. [Crossref] [PubMed]
- Lee JJ, Protheroe CA, Luo H, et al. Eosinophil-dependent skin innervation and itching following contact toxicant exposure in mice. J Allergy Clin Immunol 2015;135:477-87. [Crossref] [PubMed]
- Naidoo K, Jagot F, van den Elsen L, et al. Eosinophils Determine Dermal Thickening and Water Loss in an MC903 Model of Atopic Dermatitis. J Invest Dermatol 2018;138:2606-16. [Crossref] [PubMed]
- Lin L, Hwang BJ, Culton DA, et al. Eosinophils Mediate Tissue Injury in the Autoimmune Skin Disease Bullous Pemphigoid. J Invest Dermatol 2018;138:1032-43. [Crossref] [PubMed]
- de Graauw E, Sitaru C, Horn M, et al. Evidence for a role of eosinophils in blister formation in bullous pemphigoid. Allergy 2017;72:1105-13. [Crossref] [PubMed]
- Simon D, Yousefi S, Cazzaniga S, et al. Mepolizumab failed to affect bullous pemphigoid: A randomized, placebo-controlled, double-blind phase 2 pilot study. Allergy 2020;75:669-72. [Crossref] [PubMed]
- Kridin K. Peripheral eosinophilia in bullous pemphigoid: prevalence and influence on the clinical manifestation. Br J Dermatol 2018;179:1141-7. [Crossref] [PubMed]
- Simon D, Simon HU. Therapeutic strategies for eosinophilic dermatoses. Curr Opin Pharmacol 2019;46:29-33. [Crossref] [PubMed]
- Germic N, Hosseini A, Yousefi S, et al. Regulation of eosinophil functions by autophagy. Semin Immunopathol 2021;43:347-62. [Crossref] [PubMed]
- Steinhoff M, Buddenkotte J, Lerner EA. Role of mast cells and basophils in pruritus. Immunol Rev 2018;282:248-64. [Crossref] [PubMed]
- Ito Y, Satoh T, Takayama K, et al. Basophil recruitment and activation in inflammatory skin diseases. Allergy 2011;66:1107-13. [Crossref] [PubMed]
- Sokol CL, Chu NQ, Yu S, et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol 2009;10:713-20. [Crossref] [PubMed]
- Mashiko S, Mehta H, Bissonnette R, et al. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J Dermatol Sci 2017;88:167-74. [Crossref] [PubMed]
- Takahagi S, Tanaka A, Hide M. Sweat allergy. Allergol Int 2018;67:435-41. [Crossref] [PubMed]
- Siracusa MC, Saenz SA, Hill DA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 2011;477:229-33. [Crossref] [PubMed]
- Wang F, Trier AM, Li F, et al. A basophil-neuronal axis promotes itch. Cell 2021;184:422-440.e17. [Crossref] [PubMed]
- Hou T, Tsang MS, Kan LL, et al. IL-37 Targets TSLP-Primed Basophils to Alleviate Atopic Dermatitis. Int J Mol Sci 2021;22:7393. [Crossref] [PubMed]
- Krystel-Whittemore M, Dileepan KN, Wood JG. Mast Cell: A Multi-Functional Master Cell. Front Immunol 2016;6:620. [PubMed]
- Cheng LE, Sullivan BM, Retana LE, et al. IgE-activated basophils regulate eosinophil tissue entry by modulating endothelial function. J Exp Med 2015;212:513-24. [Crossref] [PubMed]
- Eberle JU, Radtke D, Nimmerjahn F, et al. Eosinophils Mediate Basophil-Dependent Allergic Skin Inflammation in Mice. J Invest Dermatol 2019;139:1957-65.e2. [Crossref] [PubMed]
- Lucae S, Schmid-Grendelmeier P, Wüthrich B, et al. IgE responses to exogenous and endogenous allergens in atopic dermatitis patients under long-term systemic cyclosporine A treatment. Allergy 2016;71:115-8. [Crossref] [PubMed]
- Suárez-Fariñas M, Dhingra N, Gittler J, et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol 2013;132:361-70. [Crossref] [PubMed]
- Freire PC, Muñoz CH, Stingl G. IgE autoreactivity in bullous pemphigoid: eosinophils and mast cells as major targets of pathogenic immune reactants. Br J Dermatol 2017;177:1644-53. [Crossref] [PubMed]
- Messingham KN, Crowe TP, Fairley JA. The Intersection of IgE Autoantibodies and Eosinophilia in the Pathogenesis of Bullous Pemphigoid. Front Immunol 2019;10:2331. [Crossref] [PubMed]
(English Language Editor: J. Jones)


