
Clinical application of 3D-printed patient-specific guide plate combined with computer navigation in acetabular reconstruction following resection of periacetabular tumors
Introduction
The resection of primary periacetabular tumors and subsequent reconstruction procedures have traditionally been challenging due to the complex anatomical structure and deep location as well as the rich blood supply of the tumors. Before the 1980’s, hemipelvic amputation was the standard procedure for the treatment of primary pelvic tumors; with the rapid advances in neoadjuvant chemotherapy, imaging techniques, and surgical techniques, however, limb-preserving surgery has become the mainstay of the treatment of pelvic malignancies (1-3). The en bloc resection of the tumor could largely destroy the stability and integrity of the pelvic ring, which demanded a stable pelvic and hip joint reconstruction. Regaining good hip function and maintaining pelvic stability are not easy. Currently, reconstruction of hemipelvic defects can be either biological or mechanical. Biological reconstruction enables permanent bone healing but has many unavoidable complications, including rejection, difficult wound healing, secondary deformities, and poor functional movement. Mechanical reconstruction has become more popular in recent years; however, despite its ability to provide good initial stability, a series of problems caused by component malposition seriously hinder the long-term success of the prostheses (2,4-7).
The precise reconstruction of acetabular defects is essential to achieving good long-term limb functions; however, the removal of bony landmarks after tumor resection results in the spatial drift of the contralateral pubic bone and sacrum, which largely increases the difficulty in spatial positioning of the acetabular prosthesis. Therefore, how to accurately position the acetabular rotation center, anteversion angle, and abduction angle is an important issue for orthopedic oncologists (8,9). In the past, placement of the acetabular components and adjustment of the angles were mainly dependent on the surgeon’s experience and the intraoperative fluoroscopy. Studies showed that even experienced surgeons could make mistakes in the process of reconstruction. Furthermore, the accuracy and reproducibility of reconstruction were often unsatisfactory (10-12).
In recent years, 3D-printed patient-specific guide plate (PSG) and computerized navigation (CN) have been applied clinically, with promising results in assisting pedicle screw implantation, precise correction osteotomy of limbs, precise resection of tumors, and minimally invasive percutaneous management of deep lesions (13-16). However, few reports have described PSG- and CN-assisted acetabular reconstruction for massive defect after pelvic tumor resection. At our center, we previously reported PSG alone and CN alone assisted pedicle screw-rod-acetabular cage system for pelvic ring and hip reconstruction after tumor resection with good outcomes (17). However, these two auxiliary methods have some limitations: (I) soft tissue deformation or occlusion can decrease the accuracy of guide plate positioning and (II) navigation requires prolonged operation time due to repeated registration/positioning. Thus, PSG combined with computer navigation (PSG + CN) was adopted in our center to shorten the operative time, correct the guide plate-related errors, and ensure the accuracy and safety of acetabular reconstruction.
To investigate the feasibility and accuracy of PSG + CN for acetabular positioning and pelvic reconstruction, we compared the outcomes of limb function recovery and acetabular positioning in freehand (FH), PSG, CN, and PSG + CN groups. This study introduced a novel PSG + CN-assisted acetabular position technique, providing both precision and validation in pelvic reconstruction following the resection of periacetabular tumors.
We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-7013/rc).
Methods
Inclusion and exclusion criteria
The inclusion criteria were as follows: (I) with pathologically confirmed primary malignant tumor in the pelvis; (II) underwent en bloc tumor resection and pelvic reconstruction with pedicle screw-rod-acetabular cage system; and (III) with complete computed tomography (CT) data and follow-up information.
The exclusion criteria were as follows: (I) with expected survival time less than 3 months; (II) with metastasis at the first diagnosis; (III) without complete CT data or follow-up information.
General data
The clinical records of patients (n=84) with periacetabular tumor who presented between January 2013 to December 2020 January were reviewed. All operations were performed by the same group of orthopaedic oncology surgeons. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Teaching and Research Ethics Committee of Xijing Hospital, Fourth Military Medical University (No. FMMU2019-075), and informed consent was taken from all the patients. These 84 patients were divided into 4 groups: (I) the FH group, consisting of 29 patients (13 males and 16 females) aged 42.5±5.8 years, including 10 cases of chondrosarcoma, 8 cases of osteosarcoma, 5 cases of Ewing sarcoma, 4 cases of undifferentiated pleomorphic sarcoma (UPS), 1 case of angiosarcoma, and 1 case of synovial sarcoma; (II) the PSG group, consisting of 18 patients (10 males and 8 females) aged 39.6±7.8 years, including 6 cases of chondrosarcoma, 5 cases of osteosarcoma, 3 cases of Ewing sarcoma, 2 cases of UPS, and 2 cases of synovial sarcoma; (III) the CN group, consisting of 14 patients (7 males and 7 females) aged 41.2±5.7 years, including 4 cases of chondrosarcoma, 5 cases of osteosarcoma, 2 cases of Ewing sarcoma, 2 cases of UPS, and 1 case of synovial sarcoma; and (IV) the PSG + CN group, consisting of 23 patients (11 males and 12 females) aged 39.5±6.4 years, including 8 cases of chondrosarcoma, 9 cases of osteosarcoma, 3 cases of Ewing sarcoma, 1 case of UPS, 1 case of leiomyosarcoma, and 1 case of synovial sarcoma. The demographic and clinical characteristics of all patients were detailed in Table 1.
Table 1
| Item | Results | n | Percentage (%) |
|---|---|---|---|
| Age (years) | <55 | 73 | 86.9 |
| ≥55 | 11 | 13.1 | |
| Gender | Male | 41 | 48.8 |
| Female | 43 | 51.2 | |
| BMI (kg/m2) | <24 | 69 | 82.1 |
| ≥24 | 15 | 17.9 | |
| Chemotherapy | Yes | 26 | 31.0 |
| No | 58 | 69.0 | |
| Radiotherapy | Yes | 9 | 10.7 |
| No | 75 | 89.3 | |
| Resection type | Involving zone I + IV | 31 | 36.9 |
| Not involving zone I + IV | 53 | 63.1 | |
| Surgical procedures | FH | 29 | 34.5 |
| PSG | 18 | 21.4 | |
| CN | 14 | 16.7 | |
| PSG + CN | 23 | 27.4 | |
| Survival status | Tumor-free survival | 56 | 66.7 |
| Survival with tumor | 13 | 15.5 | |
| Death | 15 | 17.8 | |
| Complications | Incision | 18 | 21.4 |
| Prothesis | 16 | 19.1 | |
| No | 50 | 59.5 |
BMI, body mass index; FH, freehand; PSG, 3D-printed patient-specific guide plate; CN, computer navigation.
Treatment
All patients were diagnosed by preoperative biopsy and underwent pelvic radiography, thin-section CT, magnetic resonance imaging (MRI), and CT angiography to determine the extent and site of the tumor invasion. Embolization of feeding vessels of the tumor was performed 1 to 3 days before surgery to reduce intraoperative bleeding. A conventional combined ilioinguinal–iliofemoral approach was used to mobilize and protect the iliac neurovascular bundle, male spermatic cord, and ureter. The lateral pelvis was separated from the gluteal muscle, and the medial pelvis was separated from the iliacus muscle; meanwhile, the tumor was wrapped by the normal muscle cuff. The tensor muscle of the broad fascia and the rectus femoris gap were separated along the iliofemoral incision. The reflected head of the rectus femoris was divided to expose and dissect the hip capsule; the head and neck of the femur were osteotomized to expose the acetabulum and ischia. The muscle insertion attached to ischia, sacrospinous ligament and the sacrotuberous ligament was severed. Depending on the extent of tumor invasion, the tumor was en bloc removed anteriorly around the pubic symphysis or posteriorly around the ischial tuberosity.
Reconstruction in FH group
Two pedicle screws were implanted proximally to the residual ilium or S1 and S2 vertebrae, and two pedicle screws were implanted in the remaining sciatic and pubic branches or in the contralateral upper and lower pubic branches. The position of the acetabulum was determined empirically. The original axis of rotation of the femoral head was restored, the abduction angle of the acetabular cup was adjusted to 45°, and the anteversion angle was adjusted to 10–15°. The screws were attached to the acetabular cage using titanium rods. X-ray fluoroscopy was done during the operation to adjust the acetabular position for reconstruction.
Reconstruction in PSG group
Virtual surgical planning and production of 3D-printed guide plates were completed preoperatively. The reconstruction guide plate was designed according to the contralateral acetabular structure and the morphologies of the residual pelvis after tumor resection. After the STL file of the guide plate was imported into a 3D printing device (EZAU 3D printer), the guide plate was 3D-printed with polylactic acid material and then sterilized for further use. The proximal part of guide plate was fixed to residual ilium or the sacrum, and the distal part was fixed to the residual pubis or ischium. A special titanium acetabular cage was also placed into the guide plate to determine the position of the cage. Thereafter, the screws and rods were connected to the cage as stated above. Intraoperative fluoroscopy was performed to confirm the position of the acetabular prosthesis, and the guide plate was removed if the position was satisfactory (Figure 1A,1B).
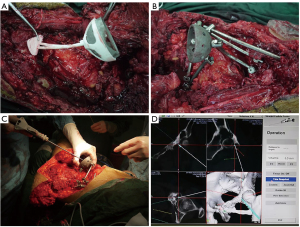
Reconstruction in CN group
Acetabulum reconstruction was performed using CN system. Registration was required prior to tumor resection and the tracker was usually fixed to the residual bone (usually the sacrum or the contralateral pubic bone). Subsequently, the operative area was spatially registered, and the accuracy of the navigation was verified by matching the intraoperative CT scanning and pre-operative CT images. The acetabular cage was placed in the original acetabular position with the help of CN. The abduction angle of acetabular cage was positioned at 45° while the anteversion angle was at 10–15° through using “precise angle measurement” function in navigation system (Figure 1C,1D).
Reconstruction in the PSG + CN group
After periacetabular tumor resection, the landmarks in hemipelvis were usually removed. Furthermore, the pelvic ring was disrupted and remnant bone had spatial drift. To decrease the spatial drift and identify new landmarks in residual bone, the proximal part of guide plate was fixed to residual ilium or the sacrum, and the distal part was fixed to the residual pubis or ischium. A special titanium acetabular cage was placed into the guide plate to determine the position of the cage. The appropriate new landmarks in residual bone were used for registration in CN system. After the initial verification of the position of acetabular cage by using guide plate, the rotation center, offset, anteversion angle, and abduction angle were further verified and fine-tuned with CN using the method described above (Figure 2, Video 1).
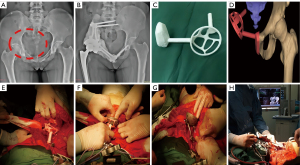
Finally, the screw-rod-acetabular cage system was reinforced with bone cement wrapping. The artificial femoral stem and a femoral head were implanted into femur and articulated with acetabular cage. Thereafter, the stable hip joint and pelvic ring were reconstructed. When the stability and mobility of joint were found to be satisfactory, the incision was closed layer by layer.
Follow-up and evaluation
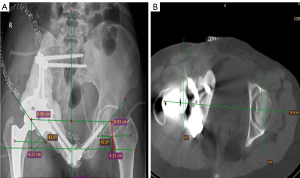
The patients were examined every 3 months for the first 2 years, then every 6 months between 2 and 5 years, and annually thereafter. The radiography and CT were performed to detect local recurrence. Chest CT scans were also required to detect metastasis. At the final follow-up visit, limb function was evaluated for with the Musculoskeletal Tumor Society (MSTS) scoring system. Four parameters were measured on CT images and radiographs using RadiAnt DICOM Viewer (Medixant, Poznań, Poland) (Figure 3). These parameters included: (I) the distance discrepancy from the rotation center to the upper edge of bilateral lesser trochanter, which were used to assess whether the lower limbs were equally long, i.e., vertical offset discrepancy (VOD); (II) distance discrepancy from the rotation center to the body vertical midline, which was used to evaluate whether the reconstructed offset was equal to the contralateral side, i.e., horizontal offset discrepancy (HOD); (III) the abduction angles discrepancy (ABAD) between the reconstructed acetabulum and the healthy side; (IV) the anteversion angle discrepancy (ANAD) between reconstructed acetabulum and the healthy side. The oncological prognosis and the complications including infections, prosthesis loosening, dislocation, and fracture were recorded.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software package (IBM Corp, Armonk, NY, USA). The comparisons among multiple groups were based on F test, and the Student-Newman-Keuls t-test was used for pairwise comparisons. The endpoint event was the occurrence of mechanical failure of the prosthesis, including aseptic loosening, dislocation, and fracture. The Kaplan-Meier method was used for analyzing the survival rates of prostheses, whereas log-rank test was applied for comparing the differences in survival rates among groups. Univariate analysis and Cox regression models were used to assess risk factors for postoperative prosthetic mechanical failure. P value of <0.05 was considered statistically significant.
Results
Surgical evaluation and functional outcomes
The operative time was 333±40, 297±27, 359±40, and 300±41 minutes in FH group, PSG group, CN group, and PSG + CN group, respectively; the intraoperative blood loss was 5,003±483, 4,756±488, 4,936±434, and 4,613±349 mL; the number of intraoperative fluoroscopy views was 17.1±2.4, 8.6±1.6, 4.6±1.1, and 1.7±0.6; the MSTS scores at the last follow-up were 20.2±2.2, 21.6±3.0, 22.72±2.2, and 24.2±2.3 points. The differences between these 4 groups were statistically significant (all P<0.05; Table 2). The results of pairwise comparisons were listed in Table 3.
Table 2
| Group | OD (min) | BL (mL) | NS | MSTS | VOD (mm) | HOD (mm) | ABAD (°) | ANAD (°) |
|---|---|---|---|---|---|---|---|---|
| FH | 333±41 | 5,003±483 | 17.1±2.4 | 20.2±2.2 | 8.4±1.9 | 9.0±1.9 | 8.6±1.8 | 5.9±1.6 |
| PSG | 297±27 | 4,756±488 | 8.6±1.6 | 21.6±3.0 | 5.9±2.2 | 6.1±2.2 | 5.6±2.0 | 3.6±1.7 |
| CN | 359±40 | 4,936±434 | 4.6±1.1 | 22.72±2.2 | 4.1±1.3 | 3.2±1.5 | 2.5±1.3 | 2.9±1.6 |
| PSG + CN | 300±41 | 4,613±349 | 1.7±0.6 | 24.2±2.3 | 2.4±1.2 | 2.1±1.1 | 1.8±0.9 | 1.9±0.9 |
| F value | 10.378 | 3.752 | 390.269 | 12.804 | 86.691 | 75.633 | 88.511 | 38.902 |
| P value | 0.000 | 0.014 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
FH, freehand; PSG, 3D-printed patient-specific guide plate; CN, computer navigation; OD, operative duration; BL, blood loss; NS, number of C-arm shots; MSTS, Musculoskeletal Tumor Society; VOD, vertical offset discrepancy; HOD, horizontal offset discrepancy; ABAD, abduction angles discrepancy; ANAD, anteversion angle discrepancy.
Table 3
| Items | I–II | I–III | I–IV | II–III | II–IV | III–IV |
|---|---|---|---|---|---|---|
| Operative duration | + | + | + | + | − | + |
| Blood loss | − | − | + | − | − | − |
| Number of C-arm shots | + | + | + | + | + | + |
| MSTS | + | + | + | − | + | − |
| VOD | + | + | + | + | + | + |
| HOD | + | + | + | + | + | − |
| ABAD | + | + | + | + | + | − |
| ANAD | + | + | + | − | + | − |
FH, freehand; PSG, 3D-printed patient-specific guide plate; CN, computer navigation; MSTS, Musculoskeletal Tumor Society; VOD, vertical offset discrepancy; HOD, horizontal offset discrepancy; ABAD, abduction angles discrepancy; ANAD, anteversion angle discrepancy. +, P<0.05; −, P>0.05. Group I: FH; Group II: PSG; Group III: CN; Group IV: PSG + CN.
Evaluation of acetabular reconstruction
Three parameters including vertical offset, horizontal offset, and the abduction angle were measured on X-ray film, whereas the anteversion angle was measured on CT image (Figure 3). The VOD was 8.4±1.9, 5.9±2.2, 4.1±1.3, and 2.4±1.2 mm in FH group, PSG group, CN group, and PSG + CN group, respectively; the HOD was 9.0±1.9, 6.1±2.2, 3.2±1.3, and 2.1±1.2 mm, correspondingly; the ABAD was 8.6°±1.8°, 5.6°±2.0°, 2.5°±1.3°, and 1.8°±0.9°, correspondingly; the ANAD was 5.9°±1.6°, 3.6°±1.7°, 2.9°±1.6°, and 1.9°±0.9°, correspondingly. These parameters showed significant difference among 4 groups (all P<0.05; Table 2). The results of pairwise comparisons were listed in Table 3.
Tumor control and complications
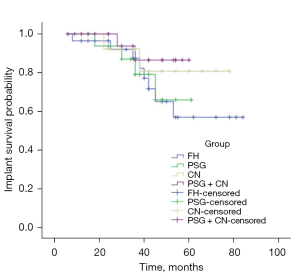
The follow-up was 45.5±21.1 months (range, 6–84 months) in FH group (n=29), in which 4 patients had local recurrence and 7 suffered from both recurrence and metastasis, among whom 5 patients died. The remaining 18 patients (18/29) had no recurrence or metastasis during the follow-up period. The follow-up was 38.6±13.8 months (range, 12–61 months) in PSG group (n=18), in which 3 patients had local recurrence and 5 suffered from both recurrence and metastasis, among whom 3 patients died. The remaining 10 patients (10/18) had no recurrence or metastasis during the follow-up period. The follow-up was 44.9±19.6 months (range, 12–78 months) in CN group (n=14), in which 2 patients had local recurrence and 4 suffered from both recurrence and metastasis, among whom 3 patients died. The remaining 8 patients (8/14) had no metastasis during the follow-up period. The follow-up was 36.4±16.3 months (range, 6–60 months) in PSG + CN group (n=23), in which 3 patients had local recurrence and 6 suffered from both recurrence and metastasis, among whom 3 patients died. The remaining 14 patients (14/23) had no recurrence or metastasis during the follow-up period. Infections were observed in 6, 4, 3, and 5 cases in FH, PSG, CN, and PSG + CN groups, respectively. Mechanical failure was defined as the loosening, dislocation, and fracture of the prosthesis. It occurred in 8, 4, 2, and 2 patients in FH, PSG, CN, and PSG + CN groups, respectively. The survival rate of the prosthesis in zone I or IV was lower than that in zones II and III (P=0.042). Body mass index (BMI) was another risk factor for mechanical failure (P=0.040), whereas age (P=0.195), gender (P=0.236), chemotherapy (P=0.756), radiotherapy (P=0.167), and assistance modalities for reconstruction (P=0.133) had no statistically significant effect on prosthesis failure (Table 4). The 2- and 5-year survival rates of prosthesis were 96.4% and 57.0% in FH group, 93.8% and 65.9% in PSG group, 92.3% and 80.8% in CN group, and 93.8% and 86.5% in PSG + CN group, respectively. No statistical difference was detected among groups (χ2=2.396, P=0.494; Figure 4).
Table 4
| Variables | Mechanical failure, n (%) | Nonmechanical failure, n (%) | P value (univariate analysis) | P value (Cox) |
|---|---|---|---|---|
| Age (years) | 0.431 | 0.195 | ||
| <55 | 13 (15.5) | 60 (71.4) | ||
| ≥55 | 3 (3.6) | 8 (9.5) | ||
| Gender | 0.273 | 0.236 | ||
| Male | 10 (11.9) | 31 (36.9) | ||
| Female | 6 (7.1) | 37 (44.1) | ||
| BMI | 0.007 | 0.040 | ||
| <24 | 9 (10.7) | 60 (71.5) | ||
| ≥24 | 7 (8.3) | 8 (9.5) | ||
| Chemotherapy | 0.977 | 0.756 | ||
| Yes | 5 (5.9) | 21 (25.0) | ||
| No | 11 (13.1) | 47 (56.0) | ||
| Radiotherapy | 0.363 | 0.167 | ||
| Yes | 3 (3.6) | 6 (7.1) | ||
| No | 13 (15.5) | 62 (73.8) | ||
| Tumor location | 0.024 | 0.042 | ||
| Involving I/IV | 10 (11.9) | 21 (25.0) | ||
| Not involving I/IV | 6 (7.1) | 4 (56.0) | ||
| Surgical procedures | 0.349 | 0.133 | ||
| FH | 8 (9.5) | 21 (25.0) | ||
| PSG | 4 (4.7) | 14 (16.7) | ||
| CN | 2 (2.4) | 12 (14.3) | ||
| PSG + CN | 2 (2.4) | 21 (25.0) |
BMI, body mass index; FH, freehand; PSG, 3D-printed patient-specific guide plate; CN, computer navigation.
Discussion
Reconstruction of the hemipelvis after resection of periacetabular tumors remains a particularly challenging procedure. Despite the possibility of local recurrence, limb-sparing treatment is still recommended as it provides a better quality of life (18). Reconstruction with a mechanical prosthesis (e.g., custom-made prostheses, saddle prostheses, modular prostheses, and screw-rod-acetabular cage prostheses) is the main limb-sparing approach. Surgeries with saddle prostheses and modular prostheses are highly indicated; however, inadequate residual bone cannot provide enough support, which leads to a high rate of prosthetic loosening. The custom-made titanium prostheses can achieve precise fitness with the residual bone. However, it was not widely used due to complex design, time-consuming preparation, and affordability.
Reconstruction with screw-rod-acetabular cage prostheses is a common option because it can be performed for any type of pelvic defect without additional complex preoperative customization (19-22). However, regardless of the promising results, this prosthesis did have some limitations, especially aseptic loosening, and broken hardware in long-term follow-up. This was largely because of intra-operative malposition of prosthesis. In our center, the PSG + CN technique had been adopted to achieve precise positioning of the acetabulum, which was particularly difficult for conventional approaches. Here, we report on the application of PSG + CN in a series of patients undergoing pelvic tumor resection and reconstruction, and provide a comprehensive comparison with conventional surgical approach.
Precise intraoperative positioning and timely imaging feedback are highly helpful for surgeons, especially in procedure of prosthesis placement (23). As shown in current study, the CN group outperformed the FH group in terms of the number of fluoroscopic views, position of acetabular prosthesis, and limb function at the final follow-up. The availability of PSG offers a new solution to the challenge of intraoperative acetabular positioning and reconstruction, which have recently been described as an alternative in replicating surgical plans in bone tumor surgery (24). Compared with FH, PSG has the following advantages: (I) it can reduce the additional time and number of fluoroscopic views required for acetabular cage position and incidence of complications; (II) the guide plate is designed according to the preoperative osteotomy plan, which can ensure the safe margin for tumor resection.
In our study, results showed that CN group had longer operative times than PSG group likely as a result of the following: (I) it usually takes long time to find appropriate bony landmarks after tumor resection; and (II) repeated registration and verification was usually required, which prolonged the operation. On the other hand, compared with CN assisted position, the position of the guide plate might easily drift for a variety of reasons. Firstly, the guide plate is prone to slightly deform during sterilization and packaging. Secondly, jamming and deformation between the guide plate and tissue is common when placing guide; Thirdly, the guide plate is designed according to the structure of the bone tissue but neglecting the thick cartilage layer. Therefore, intraoperative application of guide plate requires that the soft tissue be stripped as much as possible while preserving adequate bony structures.
Based on the encouraging results of previous studies, we attempted to combine PSG and CN technique for more accurate and efficient reconstruction. The complementary use of PSG provided the correct anatomical positioning. CN with submillimeter registration error might provide an objective assessment to confirm the correct placement of PSG. Eliminating the need for initial positioning with CN notably shortened the operation time. Use of PSG was reported to improve the efficiency of reconstruction and also reduce operation time (15,23-25). Theoretically, additional fluoroscopy was not needed during PSG + CN. However, 1 or 2 sessions of intraoperative fluoroscopy might be feasible. Although PSG was widely used, positioning drift still existed in complex anatomical sites (such as the pelvis) because the cartilage layer prevented the guide plate from intimately fitting to the bone (26,27). Therefore, the combination of CN and later fine-tuning to PSG ensured the accuracy of acetabular positioning. Imaging measurements showed that both the offset and angle of acetabular placement angle were better in the PSG + CN group, although the differences were not statistically significant. In our study, the MSTS score of PSG + CN group at the final follow-up was 24.2±2.4, which was superior to that of CN group (22.72±2.2, P>0.05) and PSG group (21.6±3.0, P<0.05). The good outcomes could be attributed to the accurate reconstruction. There was no significant difference in disease-free survival at the final follow-up, indicating that safe margin was ensured in all 3 groups.
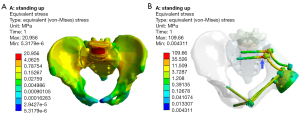
Long-term mechanical failures such as aseptic loosening, dislocation, and fracture of implanted prosthesis are inevitable (28). Zone I or IV involvement may be a potential risk factor, partly because the disruption of the sacroiliac joint causes impaired transmission and dispersion of stresses. Few finite element analyses investigated the value of hemipelvic reconstruction with screw-rod-acetabular cage system. It was explored in our study. It was found that although the pelvic mechanical transmission could be restored after pelvis reconstruction, the stresses were concentrated at the S1 and S2 screw-rod junction, the same location where stresses occurred at the sacroiliac joint of the healthy pelvis. However, the surface area of the junction is much smaller than that of the sacroiliac joint (Figure 5). BMI was another risk factor for mechanical failure although the peak stress of the prosthesis was only 109.66 MPa in the standing position, far below the yield strength of titanium (789–1,013 MPa) (29). For patients with BMI >24, the increase in internal fixation stresses contributed to fatigue fracture or loosening of the prosthesis, especially with increased intensity of physical activity (30). Studies showed that the accuracy of the acetabular prosthesis position was related to the function of the hip joint and the longevity of the prosthesis (17,23,29-31). Although there was no significant association between the precision of reconstruction and the mechanical failure of the prosthesis in our study (P>0.05), superiority was observed. The 2-year survival rates of the prosthesis were higher than 90% in FH, PSG, CN, and PSG + CN groups, but the 5-year survival rates (57.0%, 65.9%, 80.8%, and 86.5%, respectively) differed significantly. The results indicated that precise reconstruction could provide good long-term limb function and reduce the possibility of revision.
To achieve better clinical outcomes, both CN and PSG are attempted in clinical application. Leitner et al. (32) used CN to improve the accuracy of pedicle screw placement and significantly lowered the risk of pedicle perforation. By using PSG, Yang et al. (33) reduced the distance in deviations of the reconstructed maxilla or mandible and enhanced reconstruction accuracy. By using PSG + CN, Wong et al. (34) successfully implemented complex joint-preserving bone tumor resection and reconstruction to get better functions. In current study, the surgical accuracy and limb functions suggested that PSG + CN might excellently replicate the surgical planning. However, despite the prominent advantages of CN and PSG, there are still several difficulties need to be overcome: (I) the limitations of CN, such as high costs of system, long learning curve, and complex intraoperative assembly, need to be addressed; (II) the cost-effectiveness of PSG must be considered, as the cost, manpower, and time spent on additional design and manufacturing processes are higher than those of FH; and (III) the design, manufacturing, and application of the guide plate require additional personnel with a multidisciplinary background.
Conclusions
PSG + CN for acetabular positioning and reconstruction fully embodies the principles of precise and personalized treatment, avoiding both the time-consuming and tedious process of CN alone and the drift errors caused by PSG alone. It has potential advantages in improving the accuracy and safety of reconstruction following periacetabular tumor resection.
Acknowledgments
Funding: The study was supported by National Key Research and Development program of China (2016YFB1101104), Key Research and Development program of Shaanxi Province, China (2018ZDXM-SF-075).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-7013/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-7013/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-7013/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Teaching and Research Ethics Committee of Xijing Hospital, Fourth Military Medical University (No. FMMU2019-075), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jansen JA, van de Sande MA, Dijkstra PD. Poor long-term clinical results of saddle prosthesis after resection of periacetabular tumors. Clin Orthop Relat Res 2013;471:324-31. [Crossref] [PubMed]
- Gebert C, Wessling M, Hoffmann C, et al. Hip transposition as a limb salvage procedure following the resection of periacetabular tumors. J Surg Oncol 2011;103:269-75. [Crossref] [PubMed]
- Mankin HJ, Hornicek FJ. Internal hemipelvectomy for the management of pelvic sarcomas. Surg Oncol Clin N Am 2005;14:381-96. [Crossref] [PubMed]
- Cottias P, Jeanrot C, Vinh TS, et al. Complications and functional evaluation of 17 saddle prostheses for resection of periacetabular tumors. J Surg Oncol 2001;78:90-100. [Crossref] [PubMed]
- Satcher RL Jr, O'Donnell RJ, Johnston JO. Reconstruction of the pelvis after resection of tumors about the acetabulum. Clin Orthop Relat Res 2003;209-17. [Crossref] [PubMed]
- Delloye C, Banse X, Brichard B, et al. Pelvic reconstruction with a structural pelvic allograft after resection of a malignant bone tumor. J Bone Joint Surg Am 2007;89:579-87. [Crossref] [PubMed]
- Wang J, Tang Q, Xie X, et al. Iliosacral resections of pelvic malignant tumors and reconstruction with nonvascular bilateral fibular autografts. Ann Surg Oncol 2012;19:4043-51. [Crossref] [PubMed]
- Fujiwara T, Lex JR, Stevenson JD, et al. Surgical treatment for pelvic Ewing sarcoma: What is a safe and functional acetabular reconstruction when combined with modern multidisciplinary treatments? J Surg Oncol 2019;120:985-93. [Crossref] [PubMed]
- Ji T, Guo W, Yang RL, et al. Modular hemipelvic endoprosthesis reconstruction--experience in 100 patients with mid-term follow-up results. Eur J Surg Oncol 2013;39:53-60. [Crossref] [PubMed]
- Gouin F, Paul L, Odri GA, et al. Computer-Assisted Planning and Patient-Specific Instruments for Bone Tumor Resection within the Pelvis: A Series of 11 Patients. Sarcoma 2014;2014:842709. [Crossref] [PubMed]
- Liang H, Ji T, Zhang Y, et al. Reconstruction with 3D-printed pelvic endoprostheses after resection of a pelvic tumour. Bone Joint J 2017;99-B:267-75. [Crossref] [PubMed]
- Yu AW, Duncan JM, Daurka JS, et al. A feasibility study into the use of three-dimensional printer modelling in acetabular fracture surgery. Adv Orthop 2015;2015:617046. [Crossref] [PubMed]
- Jentzsch T, Vlachopoulos L, Fürnstahl P, et al. Tumor resection at the pelvis using three-dimensional planning and patient-specific instruments: a case series. World J Surg Oncol 2016;14:249. [Crossref] [PubMed]
- Sallent A, Vicente M, Reverté MM, et al. How 3D patient-specific instruments improve accuracy of pelvic bone tumour resection in a cadaveric study. Bone Joint Res 2017;6:577-83. [Crossref] [PubMed]
- Wang F, Zhu J, Peng X, et al. The application of 3D printed surgical guides in resection and reconstruction of malignant bone tumor. Oncol Lett 2017;14:4581-4. [Crossref] [PubMed]
- Laitinen MK, Parry MC, Albergo JI, et al. Is computer navigation when used in the surgery of iliosacral pelvic bone tumours safer for the patient? Bone Joint J 2017;99-B:261-6. [Crossref] [PubMed]
- Guo Z, Li J, Pei GX, et al. Pelvic reconstruction with a combined hemipelvic prostheses after resection of primary malignant tumor. Surg Oncol 2010;19:95-105. [Crossref] [PubMed]
- Ozaki T, Hillmann A, Bettin D, et al. High complication rates with pelvic allografts. Experience of 22 sarcoma resections. Acta Orthop Scand 1996;67:333-8. [Crossref] [PubMed]
- Uchida A, Myoui A, Araki N, et al. Prosthetic reconstruction for periacetabular malignant tumors. Clin Orthop Relat Res 1996;238-45. [Crossref] [PubMed]
- Gradinger R, Rechl H, Hipp E. Pelvic osteosarcoma. Resection, reconstruction, local control, and survival statistics. Clin Orthop Relat Res 1991;149-58. [PubMed]
- Abudu A, Grimer RJ, Cannon SR, et al. Reconstruction of the hemipelvis after the excision of malignant tumours. Complications and functional outcome of prostheses. J Bone Joint Surg Br 1997;79:773-9. [Crossref] [PubMed]
- Krieg AH, Lenze U, Gaston MS, et al. The outcome of pelvic reconstruction with non-vascularised fibular grafts after resection of bone tumours. J Bone Joint Surg Br 2010;92:1568-73. [Crossref] [PubMed]
- Wong KC, Kumta SM, Chiu KH, et al. Computer assisted pelvic tumor resection and reconstruction with a custom-made prosthesis using an innovative adaptation and its validation. Comput Aided Surg 2007;12:225-32. [Crossref] [PubMed]
- Liu X, Liu Y, Lu W, et al. Combined Application of Modified Three-Dimensional Printed Anatomic Templates and Customized Cutting Blocks in Pelvic Reconstruction After Pelvic Tumor Resection. J Arthroplasty 2019;34:338-45.e1. [Crossref] [PubMed]
- Wang B, Xie X, Yin J, et al. Reconstruction with modular hemipelvic endoprosthesis after pelvic tumor resection: a report of 50 consecutive cases. PLoS One 2015;10:e0127263. [Crossref] [PubMed]
- Yi C, Zheng J, Li R, et al. Preliminary proposal: a classification system for reconstruction with autologous femoral head after periacetabular tumors resection. J Orthop Surg Res 2021;16:119. [Crossref] [PubMed]
- Wang J, Min L, Lu M, et al. What are the Complications of Three-dimensionally Printed, Custom-made, Integrative Hemipelvic Endoprostheses in Patients with Primary Malignancies Involving the Acetabulum, and What is the Function of These Patients? Clin Orthop Relat Res 2020;478:2487-501. [Crossref] [PubMed]
- Jeys LM, Kulkarni A, Grimer RJ, et al. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am 2008;90:1265-71. [Crossref] [PubMed]
- Ozaki T, Hoffmann C, Hillmann A, et al. Implantation of hemipelvic prosthesis after resection of sarcoma. Clin Orthop Relat Res 2002;197-205. [Crossref] [PubMed]
- Kim D, Lim JY, Shim KW, et al. Sacral Reconstruction with a 3D-Printed Implant after Hemisacrectomy in a Patient with Sacral Osteosarcoma: 1-Year Follow-Up Result. Yonsei Med J 2017;58:453-7. [Crossref] [PubMed]
- Dahake SW, Kuthe AM, Mawale MB, et al. Applications of medical rapid prototyping 312 assisted customized surgical guides in complex surgeries. Rapid Prototyping J 2016;22:934-46. [Crossref]
- Leitner L, Bratschitsch G, Sadoghi P, et al. Navigation versus experience: providing training in accurate lumbar pedicle screw positioning. Arch Orthop Trauma Surg 2019;139:1699-704. [Crossref] [PubMed]
- Yang WF, Choi WS, Wong MC, et al. Three-Dimensionally Printed Patient-Specific Surgical Plates Increase Accuracy of Oncologic Head and Neck Reconstruction Versus Conventional Surgical Plates: A Comparative Study. Ann Surg Oncol 2021;28:363-75. [Crossref] [PubMed]
- Wong KC, Sze LKY, Kumta SM. Complex joint-preserving bone tumor resection and reconstruction using computer navigation and 3D-printed patient-specific guides: A technical note of three cases. J Orthop Translat 2021;29:152-62. [Crossref] [PubMed]
(English Language Editor: J. Gray)


