
The efficacy of 99mTc-rituximab as a tracer for sentinel lymph node biopsy in cutaneous melanoma patients
Introduction
A sentinel lymph node biopsy (SLNB) is a vital procedure in melanoma patients as recommended by the National Comprehensive Cancer Network (NCCN) guideline (1). It is a key staging procedure for patients with clinical stage I-II melanoma according to the presence or absence of nodal metastasis. The available evidence strongly supports the sentinel lymph node (SLN) status as the most powerful independent factor for predicting survival (2-4). Accurate staging is generally accepted as the basis for counseling, therapeutic decision-making, and prognostication in melanoma. Depending on a variety of factors, 5–40% patients will be detected as SLN-positive (1,2,5-8). The commonly acknowledged risk factors of a positive SLN include the Clark level, mitotic rate, ulceration, lymphovascular invasion, anatomic site, tumor infiltrating lymphocytes, and primary tumor thickness (1,3,9-14). Compared to Caucasian patients, Chinese melanoma patients tend to be associated with a longer delay to diagnosis and a higher proportion of T-stage III–IV status (15,16). Therefore, an accurate SLNB procedure is particularly essential for clinical work.
The NCCN-recommended technique for SLNB consists of preoperative dynamic lymphoscintigraphy, intraoperative identification using isosulfan blue or methylene blue dye, and a gamma probe to detect radiolabeled lymph nodes (1). However, recent studies have increasingly indicated that the use of blue dye is associated with a negative outcome. Ranson et al. performed SLNB in 537 patients using both a 99mTc radiocolloid and blue dye, and reported that if the blue dye was excluded and only hot nodes were harvested, it would only result in a 5% reduction of dissected nodes without any compromise in the sensitivity of the test (17). van der Ploeg et al. retrospectively assessed 681 patients, and discovered that a blue, nonradioactive sentinel node was removed in only 0.9% of patients (18). There was a minor reduction in the collected nodes; however, there are other negative impacts of using blue dye, including anaphylaxis [ranging from 0.1% to 2.7% (19-21)], prolonged cutaneous staining (22), and its contraindication in pregnant women. In 2013, the US Food and Drug Administration approved Lymphoseek (99mTc-tilmanocept) as a radiotracer for detecting SLN in breast and melanoma patients (23). Inspired by this invention, we developed 99mTc-labeled rituximab as a novel radiotracer. The accuracy and specificity of this tracer has been tested and verified in breast cancer patients in our institution, with a sensitivity 97.4% and specificity of 100% (24,25). But its efficacy in melanoma has not been proved.
Rituximab is an antibody that specifically targets the CD20 molecules expressed in B lymphocytes. Theoretically, 99mTc-rituximab can combine with B lymphocytes in the first lymph node on the lymph circulating route, which is the precise definition of “sentinel lymph node”, and avoids imaging of second-tier lymph nodes. Because this radio tracer can be detected within at least 6–8 h post-injection, surgeons are guaranteed to have sufficient time during the operation. Besides, the side effects of blue dye, such as anaphylaxis and skin staining, can be avoided. In this study, we aimed to report the feasibility, effectiveness, and safety of using 99mTc-rituximab for the clinical testing of SLN in melanoma.
We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-6890/rc).
Methods
Patients
From February 2009 to March 2019, a total of 502 consecutive patients with melanoma underwent a biopsy at the Department of Bone and Soft Tissue Sarcoma of Peking University Cancer Hospital. The indications for SLNB included: (I) primary melanoma, Breslow thickness ≥1 mm, or Breslow thickness <1 mm and with other adverse features (e.g., ulceration, high mitotic rate, and lymphovascular invasion); (II) clinically negative lymph nodal basin (examined by palpation and ultrasound); (III) absence of distant metastasis [confirmed by routine physical examination, chest and abdominal computed tomography (CT) scan, or positron emission tomography (PET)-CT]; and (IV) feasibility for anesthesia. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Peking University Cancer Hospital and informed consent was taken from all the patients.
SLNB
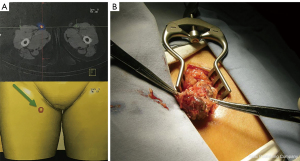
The radiotracer was prepared as described in a previous study (24). The radiotracer solution was approximately 74 MBq/mL with saline. All participants were administered with peritumoral intradermal injections of 99mTc-rituximab (10.0 MBq, 0.2 mL) on the same day or the day prior to the operation. Planar lymphoscintigraphy was then acquired by a single-photon emission computed tomography-computed tomography (SPECT-CT) system (E. Cam; Siemens, Erlangen, Germany) to determine the location and number of SLNs (Figure 1A). During the operation, the precise position of the SLNs was located using a handled gamma detection probe (Neoprobe, Dublin, OH, USA or Crystal Photonics, Berlin, Germany) before the surgical incision, after which a minimal incision (2–4 cm) was performed (Figure 1B). All radioactive nodes with a counting rate ≥10% of the hottest node were removed.
Pathology
During routine pathologic examination, SLNs were cut along the major axis, formalin-fixed, embedded in paraffin, and stained with hematoxylin and eosin (H&E). Intraoperative frozen section investigations were not performed. Paraffin-embedded specimens were examined by light microscopy at magnifications of ×40 and ×200. Additional immunohistochemical (IHC) staining (S100, HMB-45 and Melan-A) was used for suspicious cases identified with H&E. All negative SLNs with local nodal recurrence were retrospectively reviewed by an experienced pathologist (Lai). Additional serial sectioning (at a 2-mm interval) was performed on all of the reviewed SLN biopsy specimens, and doubtful cases were stained for IHC.
Statistical analysis
For the statistical analysis of the data, contingency tables were analyzed using a χ2 test. For the survival analysis, a Kaplan-Meier estimator was used with generalized Wilcoxon and log-rank tests for bivariate comparisons. In the analysis of risk factors, binary logistic regression was deployed. The disease-free survival (DFS) time was calculated from the time of the primary diagnosis to the time point of disease recurrence or death due to any reason. The overall survival (OS) time was calculated from the time of primary diagnosis to the time point of death for any reason. All statistical analyses were performed using SPSS 25.0 (SPSS Inc., Chicago, IL, USA). The differences between groups were considered to be significant for P values <0.05.
Results
A total of 502 patients were enrolled in this study, among whom 239 were men and 263 were women. Acral malignant melanoma (MM) and T3–4 stage patients comprised the majority, accounting for 74.5% (374 patients) and 59.4% (298 patients), respectively (Table 1). The SPECT-CT scanning was omitted in 3 participants due to patient rejection or an operation being pressed for time. We detected SLNs in the remaining 499 participants (100%). The imaging time ranged from 0.33 to 16 h (median: 1 h). The number of imaged SLNs ranged from 1–8 (median 1). During the operation, SLNs were successfully harvested from all participants, including the 3 patients without SPECT-CT results. The average number of harvested SLN was 2.75.
Table 1
| Characteristics | Total patients | Number of SLN-positive patients (%) |
|---|---|---|
| Patient(n) | 502 | 122 (24.3) |
| Male | 239 | 58 (24.3) |
| Female | 263 | 64 (24.3) |
| Age [median] | 58.0 [8–86] | 60.0 [8–80] |
| Location | ||
| Acral MM | 374 | 87 (23.3) |
| Upper limb | 91 | 15 (16.5) |
| Lower limb | 283 | 72 (25.4) |
| Cutaneous MM | 128 | 35 (27.3) |
| Upper limb | 21 | 5 (23.8) |
| Lower limb | 58 | 13 (22.4) |
| Trunk | 45 | 16 (35.6) |
| Others (perineum, head, etc.) | 4 | 1 (25.0) |
| T stage | ||
| Tis | 5 | 0 (0.0) |
| 1 | 65 | 9 (13.8) |
| 1a | 46 | 6 (13.0) |
| 1b | 18 | 3 (16.7) |
| 1x | 1 | 0 (0.0) |
| 2 | 95 | 15 (15.8) |
| 2a | 63 | 10 (15.9) |
| 2b | 28 | 5 (17.9) |
| 2x | 4 | 0 (0.0) |
| 3 | 137 | 37 (27.0) |
| 3a | 70 | 21 (30.0) |
| 3b | 63 | 15 (23.8) |
| 3x | 4 | 1 (25.0) |
| 4 | 161 | 52 (32.3) |
| 4a | 47 | 12 (25.5) |
| 4b | 108 | 37 (34.3) |
| 4x | 6 | 3 (50.0) |
| NA | 33 | 9 (27.3) |
| Ulceration | ||
| Positive | 216 | 58 (26.9) |
| Negative | 255 | 53 (20.8) |
| NA | 31 | 11 (35.5) |
| Lymphovascular invasion | ||
| Positive | 27 | 9 (33.3) |
| Negative | 365 | 91 (25.2) |
| NA | 110 | 22 (20.0) |
| Clark | ||
| I | 3 | 0 (0.0) |
| II | 11 | 3 (27.3) |
| III | 41 | 6 (14.6) |
| IV | 157 | 35 (22.3) |
| V | 53 | 16 (30.2) |
| NA | 237 | 62 (26.2) |
SLN, sentinel lymph node; NA, not available; MM, malignant melanoma.
Complications were observed in 32 patients (6.3%): 26 were seroma (5.2%), 6 were wound infections or lymphangitis (1.2%), and 4 were sensory nerve injuries (0.8%). The inguinal region was a high-risk region for SLNB complications compared to the axilla and neck (Table 2). There were 19 (73.1%) participants with seroma that did not require any intervention, and the remaining 7 (26.9%) participants recovered well after suctioning fluid with a syringe. Those with a wound infection or lymphangitis were treated with antibiotics. Participants with sensory nerve injures were observed and the symptoms were fully or partially relieved within 6 months. No drainage tube needed to be indwelled, and no second operation was performed.
Table 2
| Complications | N (%) | P value |
|---|---|---|
| Total patients with complications | 32/502 (6.3) | |
| Seroma | 26 (5.2) | |
| Wound infection | 6 (1.2) | |
| Sensory nerve injury | 4 (0.8) | |
| Location | ||
| Inguina | 30/387 | P<0.05 (χ2 test) |
| Axilla | 2/112 | |
| Neck | 0/3 |
SLNB, sentinel lymph node biopsy.
A total of 112 participants were diagnosed as SLN-positive after a pathological examination, in which 74 had only 1 positive SLN, 27 had 2 positive SLNs, and 11 had 3 or more positive SLNs. The SLN positive rates of the acral and cutaneous groups were not significantly different (23.3% vs. 27.3%; P=0.40). A total of 85 patients received a complete lymph node dissection. Additional positive lymph nodes (non-SLN positive) were discovered in 28 (32.9%) patients. There were 16 (57.1%) participants who had only 1 non-SLN positive lymph node, 5 (17.9%) had 2 non-SLNs positive lymph nodes, and 7 (25.0%) had 3 nodes or more.
A total of 390 patients were initially diagnosed to be SLN-negative. We followed these patients for up to 140 months (3–40 months; median: 24.0 months). There were 44 patients who exhibited local nodal basin recurrence. After a pathological and medical history review, 10 participants were found to be initially misdiagnosed as SLN-negative, and 6 participants had in-transit recurrence before nodal basin metastasis. After these 16 participants had been excluded, 28 participants were confirmed to be false-negative (FN). The FN rate was defined as the proportion of patients with nodal recurrences after negative SLNB in the patients with nodal involvement. The failure rate was defined as the number of FN patients divided by the total number of SLN-negative patients. Based on this definition, the FN rate of our tracer was 18.7%, and the failure rate was 7.2%.
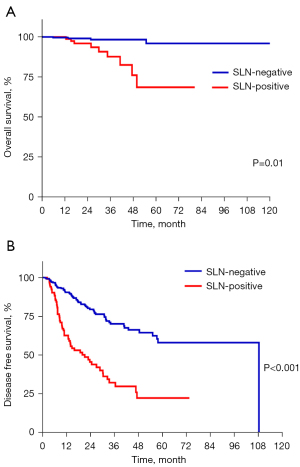
After pathological review, we increased the number of SLN-positive patients from 112 (22.3%) to 122 (24.2%), and the number of SLN-negative patients was amended to 380. We then compared the survival rate between the 2 groups. The mean DFS and OS of the SLN-negative group was 74.4 months [95% confidence interval (CI): 67.0 to 81.9] and 106.1 months (95% CI: 103.2 to 109.0), respectively. The mean DFS and OS of the SLN-positive group was 28.2 months (95% CI: 22.8 to 33.7) and 67.2 (95% CI: 59.2 to 75.1). Statistical differences were identified between the 2 groups regarding both DFS and OS rates (P<0.05) (Figure 2). These results confirmed that the SLN status was a strong predictor of survival.
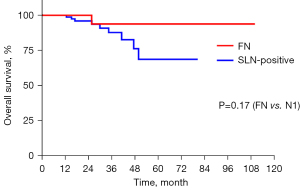
We further explored the risk factors of FN status. age, gender, T stage, ulceration, Clark stage, mitosis rate, lymphovascular invasion, and primary lesion location (acral or non-acral) were taken into account in the analysis. The FN group was compared with SLN-negative group and SLN-positive group. The results showed that T stage was the only predicting factor for the FN group compared with the SLN-negative group (OR: 1.77; 95% CI: 1.12 to 2.78; P=0.01). Acral or cutaneous subtype was not a risk factor in either comparison (FN vs. SLN-negative, P=0.33; FN vs. SLN-positive, P=0.72). There were no differences between the FN group and SLN-positive group in the listed risk factors (Table 3). The median time to nodal basin recurrence in the FN group was 10.6 months. Although misdiagnosed, the OS of FN group (mean: 94.9 months; 95% CI: 84.8 to 104.9) was not statistically different from the OS of the SLN-positive group (P=0.17) (Figure 3).
Table 3
| FN | SLN-negative | SLN-positive | P value (FN vs. SLN-negative) |
P value (FN vs. SLN-positive) |
|
|---|---|---|---|---|---|
| Patient (n) | 28 | 352 | 122 | ||
| Male | 14 | 167 | 58 | 0.79 | 0.81 |
| Female | 14 | 185 | 64 | ||
| Age [median] | 60.0 [27–84] | 58.0 [20–86] | 60.0 [8–80] | 0.91 | 0.95 |
| Location | 0.33 | 0.72 | |||
| Acral MM | 19 | 268 | 87 | ||
| Cutaneous MM | 9 | 84 | 35 | ||
| T stage | 0.01 | 0.75 | |||
| Tis | 0 | 5 | 0 | ||
| 1 | 0 | 56 | 9 | ||
| 2 | 5 | 76 | 15 | ||
| 3 | 8 | 92 | 37 | ||
| 4 | 12 | 97 | 52 | ||
| NA | 3 | 26 | 9 | ||
| Ulceration | 0.64 | 0.70 | |||
| Positive | 13 | 145 | 58 | ||
| Negative | 14 | 188 | 53 | ||
| NA | 1 | 19 | 11 | ||
| Lymphovascular invasion | 0.32 | 0.84 | |||
| Positive | 3 | 15 | 9 | ||
| Negative | 20 | 254 | 91 | ||
| NA | 5 | 83 | 22 | ||
| Clark | 0.42 | 0.98 | |||
| I | 0 | 3 | 0 | ||
| II | 0 | 8 | 3 | ||
| III | 0 | 31 | 6 | ||
| IV | 6 | 110 | 35 | ||
| V | 1 | 33 | 16 | ||
| NA | 21 | 164 | 62 |
FN, false negative; SLN, sentinel lymph node; MM, malignant melanoma; NA, not available.
Discussion
In the present study, SLN was detected in 100% of patients by SPECT-CT, and none of the participants failed the SLN biopsy. The mean number of harvested SLNs was 2.75. The operation procedure was simplified because the location and number of SLN could be identified based on the SPECT-CT report. 99mTc-rituximab has been deployed successfully as a tracer in breast cancer patients. In a study about evaluation of 99mTc-rituximab in breast cancer published in 2016, the success rate of lymphoscintigraphy and SLNB was 98.8% and 99.9% (24). Our result is in accordance with the previous study. In addition, by using the γ-detector to accurately locate the SLN before incision, surgeons could minimalize the surgical wound and shorten the operation time. Without a drainage tube, only 6.3% patients experienced postoperative complications, which was a relatively low rate compared to that of other studies (5–10%) (26-32). Most of the complications were mild and recovered following conservative treatment. Complications were more common in groin SLNB than in the axilla and neck. There results were similar to those of previous studies.
In this study, SLN was a strong predictor of both OS and DFS. These results complied with previous studies, and showed that the tracer, 99mTc-rituximab, could distinguish the SLN-positive patients from the entire population.
After revision, 122 participants (24.2%) were diagnosed as SLNB-positive, 85 of whom received a complete lymph node dissection (CLND). A total of 28 participants (32.9%) were found to have non-SLN positive lymph nodes. The non-SLN positive rate was much higher compared with the NCCN guidelines and the findings of other studies (12–32%, average 20%) (1,33-37). These differences may be attributed to a higher proportion of acral melanoma and a higher number of T3-4 patients in our group. Previous studies have demonstrated that Asian melanoma patients tend to be affected by the acral subtype, have a more advanced stage at the time of diagnosis, and face a poorer prognosis (16,38-42). These results may imply that CLND is still a necessary procedure for SLNB-positive patients with acral melanoma; thus, further randomized clinical trials are warranted.
A total of 10 participants were found to be SLN-positive following a pathological review. The NCCN guidelines for cutaneous melanoma recommended that large lymph nodes might be bisected or sliced at 2-mm intervals (1). Previously, the routine at our center was to bisect the SLN along the longitudinal axis. In the 10 misdiagnosed participants, 4 had micrometastasis on the marginal sinus of the previous SLN pathology slide; 5 had no metastasis on the primary SLN pathology slide, but after additional sectioning, micrometastasis was discovered on high magnification; and 1 had suspicious metastasis after additional sectioning, which was confirmed by IHC staining. These results prompted that 2-mm interval sectioning, instead of a bisection, may be an essential procedure in melanoma SLN pathology. The IHC staining should be applied when suspicious lesions are observed. We have negotiated with our pathologists and agreed that the 2-mm interval sectioning should be adopted as a standard procedure afterwards. We would like to observe whether the FN rate could be further lowered by additional sectioning.
The FN rate in our study was 18.7%, which was a reasonable level compared with that of previous studies (7–20%) (5,13,35,36,43-47). In our analysis, a higher T stage was a predictive factor for the FN status. These results were in accordance with the findings of a study conducted by Nowecki, who analyzed 1,207 MM patients and found that primary tumor thickness, primary tumor ulceration, Clark stage IV/V, and histological nodular melanoma were strongly associated with FN occurrence (45). The risk factors (e.g., ulceration, Clark stage, and histological type) were not significant in this study. This might have contributed to a relatively small number of FN patients (28 vs. 57; Nowecki study). The OS between the FN group and SLN-positive group was not significant, which indicated that despite an initial misdiagnosis, the survival of FN group patients would not be impaired as long as close follow-up was ensured in each patient (48-50).
Conclusions
The findings of this study suggested that 99mTc-rituximab may be employed as a simple and safe tracer in a melanoma SLN biopsy.
Acknowledgments
Funding: This study was supported by the Science Foundation of Peking University Cancer Hospital (08-29).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-6890/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-6890/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-6890/coif). All authors report that this study was supported by the Science Foundation of Peking University Cancer Hospital. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Peking University Cancer Hospital and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coit DG, Thompson JA, Albertini MR, et al. Cutaneous Melanoma, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:367-402. [Crossref] [PubMed]
- Ferrone CR, Panageas KS, Busam K, et al. Multivariate prognostic model for patients with thick cutaneous melanoma: importance of sentinel lymph node status. Ann Surg Oncol 2002;9:637-45. [Crossref] [PubMed]
- Johnson TM, Sondak VK, Bichakjian CK, et al. The role of sentinel lymph node biopsy for melanoma: evidence assessment. J Am Acad Dermatol 2006;54:19-27. [Crossref] [PubMed]
- Doting MH, Hoekstra HJ, Plukker JT, et al. Is sentinel node biopsy beneficial in melanoma patients? A report on 200 patients with cutaneous melanoma. Eur J Surg Oncol 2002;28:673-8. [Crossref] [PubMed]
- Statius Muller MG, van Leeuwen PA, de Lange-De Klerk ES, et al. The sentinel lymph node status is an important factor for predicting clinical outcome in patients with Stage I or II cutaneous melanoma. Cancer 2001;91:2401-8. [Crossref] [PubMed]
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199-206. [Crossref] [PubMed]
- Sondak VK, Taylor JM, Sabel MS, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity: lessons learned from the generation of a probabilistic model. Ann Surg Oncol 2004;11:247-58. [Crossref] [PubMed]
- Rousseau DL Jr, Ross MI, Johnson MM, et al. Revised American Joint Committee on Cancer staging criteria accurately predict sentinel lymph node positivity in clinically node-negative melanoma patients. Ann Surg Oncol 2003;10:569-74. [Crossref] [PubMed]
- Bedrosian I, Faries MB, Guerry D 4th, et al. Incidence of sentinel node metastasis in patients with thin primary melanoma (< or = 1 mm) with vertical growth phase. Ann Surg Oncol 2000;7:262-7. [Crossref] [PubMed]
- Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 2012;30:2678-83. [Crossref] [PubMed]
- Speijers MJ, Bastiaannet E, Sloot S, et al. Tumor mitotic rate added to the equation: melanoma prognostic factors changed?: a single-institution database study on the prognostic value of tumor mitotic rate for sentinel lymph node status and survival of cutaneous melanoma patients. Ann Surg Oncol 2015;22:2978-87. [Crossref] [PubMed]
- Munsch C, Lauwers-Cances V, Lamant L, et al. Breslow thickness, clark index and ulceration are associated with sentinel lymph node metastasis in melanoma patients: a cohort analysis of 612 patients. Dermatology 2014;229:183-9. [Crossref] [PubMed]
- Lima Sánchez J, Sánchez Medina M, García Duque O, et al. Sentinel lymph node biopsy for cutaneous melanoma: a 6 years study. Indian J Plast Surg 2013;46:92-7. [Crossref] [PubMed]
- Cavanaugh-Hussey MW, Mu EW, Kang S, et al. Older Age is Associated with a Higher Incidence of Melanoma Death but a Lower Incidence of Sentinel Lymph Node Metastasis in the SEER Databases (2003-2011). Ann Surg Oncol 2015;22:2120-6. [Crossref] [PubMed]
- Chi Z, Li S, Sheng X, et al. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. BMC Cancer 2011;11:85. [Crossref] [PubMed]
- Lee HY, Chay WY, Tang MB, et al. Melanoma: differences between Asian and Caucasian patients. Ann Acad Med Singap 2012;41:17-20. [PubMed]
- Ranson JM, Pantelides NM, Pandit DG, et al. Sentinel lymph node biopsy in melanoma: Which hot nodes should be harvested and is blue dye really necessary? J Plast Reconstr Aesthet Surg 2018;71:1269-73. [Crossref] [PubMed]
- van der Ploeg IM, Madu MF, van der Hage JA, et al. Blue dye can be safely omitted in most sentinel node procedures for melanoma. Melanoma Res 2016;26:464-8. [Crossref] [PubMed]
- Leong SP, Donegan E, Heffernon W, et al. Adverse reactions to isosulfan blue during selective sentinel lymph node dissection in melanoma. Ann Surg Oncol 2000;7:361-6. [Crossref] [PubMed]
- Bézu C, Coutant C, Salengro A, et al. Anaphylactic response to blue dye during sentinel lymph node biopsy. Surg Oncol 2011;20:e55-9. [Crossref] [PubMed]
- Clarke SA, Molajo A, Powell BW. Incidence of adverse reactions to patent blue dye in melanoma sentinel node biopsy: a major UK centre experience. J Plast Reconstr Aesthet Surg 2013;66:1299-300. [Crossref] [PubMed]
- Ariyan S, Ariyan C, Farber LR, et al. Reliability of identification of 655 sentinel lymph nodes in 263 consecutive patients with malignant melanoma. J Am Coll Surg 2004;198:924-32. [Crossref] [PubMed]
- Wallace AM, Han LK, Povoski SP, et al. Comparative evaluation of [(99m)tc]tilmanocept for sentinel lymph node mapping in breast cancer patients: results of two phase 3 trials. Ann Surg Oncol 2013;20:2590-9. [Crossref] [PubMed]
- Li N, Wang X, Lin B, et al. Clinical Evaluation of 99mTc-Rituximab for Sentinel Lymph Node Mapping in Breast Cancer Patients. J Nucl Med 2016;57:1214-20. [Crossref] [PubMed]
- Wang J, Fan T, He Y, et al. 99mTc-rituximab as a tracer for sentinel lymph node biopsy in breast cancer patients: a single-center analysis. Breast Cancer Res Treat 2018;168:365-70. [Crossref] [PubMed]
- Wrightson WR, Wong SL, Edwards MJ, et al. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol 2003;10:676-80. [Crossref] [PubMed]
- Chakera AH, Drzewiecki KT, Eigtved A, et al. Sentinel node biopsy for melanoma: a study of 241 patients. Melanoma Res 2004;14:521-6. [Crossref] [PubMed]
- Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg 2005;242:302-11; discussion 311-3. [Crossref] [PubMed]
- Gad D, Høilund-Carlsen PF, Bartram P, et al. Staging patients with cutaneous malignant melanoma by same-day lymphoscintigraphy and sentinel lymph node biopsy: a single-institutional experience with emphasis on recurrence. J Surg Oncol 2006;94:94-100. [Crossref] [PubMed]
- Read RL, Pasquali S, Haydu L, et al. Quality assurance in melanoma surgery: The evolving experience at a large tertiary referral centre. Eur J Surg Oncol 2015;41:830-6. [Crossref] [PubMed]
- Wasserberg N, Tulchinsky H, Schachter J, et al. Sentinel-lymph-node biopsy (SLNB) for melanoma is not complication-free. Eur J Surg Oncol 2004;30:851-6. [Crossref] [PubMed]
- de Vries M, Vonkeman WG, van Ginkel RJ, et al. Morbidity after axillary sentinel lymph node biopsy in patients with cutaneous melanoma. Eur J Surg Oncol 2005;31:778-83. [Crossref] [PubMed]
- Kettlewell S, Moyes C, Bray C, et al. Value of sentinel node status as a prognostic factor in melanoma: prospective observational study. BMJ 2006;332:1423. [Crossref] [PubMed]
- Kim C, Economou S, Amatruda TT, et al. Prognostic significance of microscopic tumor burden in sentinel lymph node in patients with cutaneous melanoma. Anticancer Res 2015;35:301-9. [PubMed]
- Rutkowski P, Szydłowski K, Nowecki ZI, et al. The long-term results and prognostic significance of cutaneous melanoma surgery using sentinel node biopsy with triple technique. World J Surg Oncol 2015;13:299. [Crossref] [PubMed]
- Bertolli E, Macedo MP, Pinto CA, et al. Metastatic area ratio can help predict nonsentinel node positivity in melanoma patients. Melanoma Res 2016;26:42-5. [Crossref] [PubMed]
- Shaw HM, Thompson JF. Frequency of nonsentinel lymph node metastasis in melanoma. Ann Surg Oncol 2002;9:934-author reply 934-5. [Crossref] [PubMed]
- Lv J, Dai B, Kong Y, et al. Acral Melanoma in Chinese: A Clinicopathological and Prognostic Study of 142 cases. Sci Rep 2016;6:31432. [Crossref] [PubMed]
- Kim HJ, Seo JW, Roh MS, et al. Clinical features and prognosis of Asian patients with acral lentiginous melanoma who have nodal nevi in their sentinel lymph node biopsy specimen. J Am Acad Dermatol 2018;79:706-13. [Crossref] [PubMed]
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin North Am 2003;83:275-82. [Crossref] [PubMed]
- Luk NM, Ho LC, Choi CL, et al. Clinicopathological features and prognostic factors of cutaneous melanoma among Hong Kong Chinese. Clin Exp Dermatol 2004;29:600-4. [Crossref] [PubMed]
- Zhang M, Zhang N. Clinical and prognostic factors in 98 patients with malignant melanoma in China. J Int Med Res 2017;45:1369-77. [Crossref] [PubMed]
- Statius Muller MG, van Leeuwen PA, van Diest PJ, et al. No indication for performing sentinel node biopsy in melanoma patients with a Breslow thickness of less than 0.9 mm. Melanoma Res 2001;11:303-7. [Crossref] [PubMed]
- Borgognoni L, Urso C, Vaggelli L, et al. Sentinel node biopsy procedures with an analysis of recurrence patterns and prognosis in melanoma patients: technical advantages using computer-assisted gamma probe with adjustable collimation. Melanoma Res 2004;14:311-9. [Crossref] [PubMed]
- Nowecki ZI, Rutkowski P, Nasierowska-Guttmejer A, et al. Survival analysis and clinicopathological factors associated with false-negative sentinel lymph node biopsy findings in patients with cutaneous melanoma. Ann Surg Oncol 2006;13:1655-63. [Crossref] [PubMed]
- Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med 2014;370:599-609. [Crossref] [PubMed]
- Yamamoto M, Fisher KJ, Wong JY, et al. Sentinel lymph node biopsy is indicated for patients with thick clinically lymph node-negative melanoma. Cancer 2015;121:1628-36. [Crossref] [PubMed]
- Satzger I, Meier A, Zapf A, et al. Is there a therapeutic benefit of complete lymph node dissection in melanoma patients with low tumor burden in the sentinel node? Melanoma Res 2014;24:454-61. [Crossref] [PubMed]
- Bamboat ZM, Konstantinidis IT, Kuk D, et al. Observation after a positive sentinel lymph node biopsy in patients with melanoma. Ann Surg Oncol 2014;21:3117-23. [Crossref] [PubMed]
- van der Ploeg AP, van Akkooi AC, Rutkowski P, et al. Prognosis in patients with sentinel node-positive melanoma without immediate completion lymph node dissection. Br J Surg 2012;99:1396-405. [Crossref] [PubMed]
(English Language Editor: J. Jones)


