
Successful combinatorial therapy of sirolimus and neuraminidase inhibitors in a patient with highly pathogenic avian influenza A (H5N6) virus: a case report
Introduction
In 2014, scientists identified the first human infection caused by the highly pathogenic avian influenza A (H5N6) virus in China (1). In recent years, the H5N6 virus has circulated widely among poultry, reassorted with other avian influenza virus (AIV) subtypes (2), and caused sporadic human infections with a high fatality rate (1). Although early antiviral therapy with neuraminidase inhibitors (NAIs) is associated with improved clinical outcomes in AIV infections (3), a significant number of patients have been lost to the H5N6 virus despite NAI treatment.
Drug repurposing, identifying new uses for existing drugs approved as indications for other conditions, is an attractive solution for developing potential H5N6 treatments. Sirolimus, an inhibitor of the mammalian target of rapamycin (mTOR), has been reported to reduce influenza virus protein expression and virus replication (4). In combination with oseltamivir, it has been demonstrated to improve clinical outcomes of severe H1N1-associated pneumonia (5). Therefore, sirolimus may be a promising drug for H5N6 treatment. This report outlines the first case of severe H5N6-associated pneumonia that was successfully treated with the combinatorial therapy of sirolimus and NAIs. We present the following case in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-704/rc).
Case presentation
The patient was a 22-year-old male without underlying medical conditions from Guangzhou in the Guangdong Province of China. He had a 5-year history of smoking (20 cigarettes per day) and was exposed to live poultry 2 days before illness onset. On September 24, 2018 (Day-0; Figure 1A), the patient developed high fever (40 ℃) and chills. A visit to the local hospital led to the prescription of ibuprofen and azithromycin. However, his complaints progressed over the following days, necessitating a visit to the emergency department of Sun Yat-sen Memorial Hospital, Sun Yat-sen University, on Day-3 after illness onset.
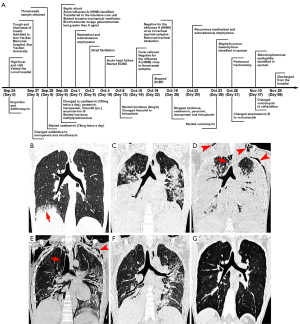
Laboratory examination upon admission showed lymphopenia and thrombocytopenia, but white blood cell count and PaO2/FiO2 values were normal (Table 1). Plain computed tomography (CT) showed patchy shadows and consolidation in the lower lobe of the right lung, which progressed rapidly to both lobes within the next 2 days (Figure 1B,1C). The patient was treated with meropenem and moxifloxacin. Considering the possibility of viral pneumonia, a throat swab sample was obtained on Day-5, which was subsequently assessed using real-time reverse transcription-polymerase chain reaction. Oseltamivir was also administered at 75 mg twice a day. The PaO2/FiO2 value declined to 84 on Day-6, while the levels of aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, creatine kinase, and myoglobin elevated. The patient developed septic shock, and the Sequential Organ Failure Assessment (SOFA) score increased from 2 to 10. On the same day, the Guangdong Provincial Center for Disease Control and Prevention identified the H5N6 virus as the source of infection within this patient.
Table 1
| Parameters | Normal range | Days after the illness onset | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 7 | 12 | 15 | 19 | 22 | 25 | 42 | 64 | ||
| White cells (×109 cells·L−1) | 3.5–9.5 | 5.2 | 3.7 | 9.0 | 11.6 | 21.1 | 17.1 | 6.51 | 11.7 | 18.1 | 9.2 |
| Neutrophil ratio (%) | 40.0–75.0 | 81.6 | 87.8 | 86.8 | 88.5 | 77.1 | 84.3 | 85.1 | 87.9 | 85.7 | 75.3 |
| Lymphocyte ratio (%) | 20.0–50.0 | 14.8 | 9.5 | 9.8 | 7.3 | 13.0 | 8.4 | 8.0 | 6.4 | 6.2 | 12.6 |
| Platelets (×109 cells·L−1) | 125–130 | 116 | 141 | 172 | 232 | 303 | 243 | 231 | 288 | 305 | 246 |
| C-reactive protein (mg·L−1) | <5.0 | – | – | – | – | 7.7 | 11.6 | 6.4 | 8.4 | 4.5 | <5.0 |
| Procalcitonin (ng·mL−1) | <0.05 | – | 3.33 | 3.75 | 0.35 | 0.13 | <0.05 | <0.05 | <0.05 | 0.24 | <0.05 |
| Alanine aminotransferase (U·L−1) | 9–50 | 17 | 48 | 29 | 198 | 85 | 69 | 64 | 97 | 122 | 61 |
| Aspartate aminotransferase (U·L−1) | 14–40 | 31 | 226 | 299 | 205 | 53 | 59 | 62 | 96 | 87 | 44 |
| Lactate dehydrogenase (U·L−1) | 108–252 | – | 1,825 | 6386 | 751 | 454 | 622 | 449 | 622 | 570 | 207 |
| Creatine kinase (U·L−1) | 126–174 | – | 8,534 | 6386 | 928 | 548 | 311 | 193 | 454 | 146 | 19 |
| Myoglobin (μg·L−1) | 28.0–72.0 | – | 409.0 | 414.9 | 524.2 | 371.7 | 81.0 | 33.5 | 44.8 | 68.9 | <21 |
| Creatinine (μmol·L−1) | 4–133 | 97 | 86 | 86 | 94 | 81 | 81 | 75 | 66 | 58 | 92 |
| PaO2/FiO2 (mmHg) | >300 | 399 | 84 | 94 | 99 | 190 | 237 | 257 | 278 | 322 | 418 |
| SOFA score | 0 | 2 | 10 | 10 | 11 | 10 | 9 | 3 | 3 | 1 | 0 |
| Throat swab virus titers (copies·mL−1) | ND | – | 8.1×105 | 9.0×105 | – | 2.1×105 | ND | ND | ND | – | – |
| Tracheal aspirate virus titers (copies·mL−1) | ND | – | – | – | 2.6×108 | 1.4×108 | 1.1×103 | 2.2×103 | ND | – | – |
SOFA, Sequential Organ Failure Assessment; ND, not detected.
The patient was then transferred to the intensive care unit (ICU) and received invasive mechanical ventilation with a low tidal volume (5 mL/kg) and an open lung ventilation strategy. The bronchoalveolar lavage fluid (BALF) galactomannan (GM) test yielded a result of greater than 5 g/mL (normal range: <0.65 µg/mL). The dosage of oseltamivir was increased to 150 mg twice a day. In addition, peramivir, amphotericin B, and low-dose corticosteroid (20 mg per day for 14 days) were also administered. Nevertheless, mediastinal and subcutaneous emphysema quickly developed on Day-7. CT images acquired on Day-10 showed a rapid progression of lung consolidation (Figure 1D). Thus, venovenous (VV) extracorporeal membrane oxygenation (ECMO) was initiated, but the patient’s condition continued to deteriorate.
On Day-12, the viral titer of the tracheal aspirate sample was still considered to be high (2.6×108 copies/mL), so sirolimus (2 mg per day for 14 days) was administered. Following this, the viral titers detected from the throat swab samples and the tracheal aspirate samples markedly decreased. On Day-16, the 4th day after sirolimus treatment was initiated, the H5N6 virus became undetectable in the throat swab specimens, and on Day-25, the 13th day after sirolimus treatment was initiated, the virus became undetectable in the tracheal aspirate samples. The patient’s clinical condition gradually improved throughout the duration of sirolimus treatment, with the PaO2/FiO2 value increasing to 278, and the SOFA score decreasing to 3. The white blood cell count increased without signs of infection. The levels of aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, creatine kinase, and myoglobin gradually decreased. ECMO was stopped on Day-22, and the endotracheal tube was removed on Day-25. Sirolimus, NAIs, and antibiotics were discontinued at the same time. CT images on Day-28 and Day-52 (Figure 1E,1F) showed the resolution of the bilateral lung infiltrations.
The patient was discharged on Day-65. Based on observations from a 2-year follow-up, he was found to be in a good condition without complications. CT images (Figure 1G) revealed a few bilateral interstitial changes in the lungs, while a pulmonary function test showed normal ventilation and slightly decreased efficiency in diffusion. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (No. SYSEC-KY-KS-2018-123). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Due to a lack of population immunity among humans and its ongoing viral evolution, the H5N6 virus poses an influenza pandemic risk if the virus were to develop more efficient transmission among humans (6). Up to 8 December 2021, a cumulative total of 53 laboratory-confirmed H5N6 patients have been reported globally, including 25 deaths (1). Among them, 49 patients required hospitalization, while 46 needed ICU treatment. The hospitalized patients usually presented with fever, sore throat, chills, cough, and myalgia as initial clinical symptoms. Their condition then quickly progressed to severe pneumonia, septic shock, and ultimately multiple organ dysfunction syndrome (MODS). The high fatality rate and rapid disease progression indicate the need for an improved treatment regimen. Drug repurposing of existing approved drugs provides an attractive alternative drug discovery strategy to identify potential treatments for H5N6.
Sirolimus, which was approved by the US Food and Drug Administration on September 15, 1999, is an immunosuppressive agent that prevents organ rejection in patients receiving renal transplants. On the cellular level, sirolimus binds to an immunophilin named FK Binding Protein-12 to generate an immunosuppressive complex. This complex inhibits the activation of mTOR, an essential member of the phosphatidylinositol 3-kinase (PI3K) family of protein kinases (7). Several studies have shown that mTOR inhibitors exhibit in vitro antiviral activity against the influenza A virus, as well as reducing both the viral protein and viral messenger RNA (mRNA) levels (4,7,8). In a lethal influenza A (H1N1 and H5N1) viral infection mouse model, the administration of everolimus (another mTOR inhibitor, 1 mg/kg/day for 14 days) significantly delayed death, in addition to reducing lung hemorrhage and increased lung weight, which are responses to infection (9). In another mouse infection model of the lethal influenza A (H1N1) pdm09 virus (pH1N1), Jia et al. showed that delayed oseltamivir-plus-sirolimus treatment protected mice by synergistically attenuating severe lung damage, reducing viral titer, and decreasing pH1N1-induced mTOR activation (10). Furthermore, in a prospective randomized, controlled study in patients with severe H1N1 pneumonia, a higher proportion of patients with sirolimus-corticosteroids-oseltamivir combinatory treatment achieved clearance of viral RNA within the airway after 7 days of treatment (75% vs. 33.3%; P<0.05) compared to those in the non-sirolimus group (5). Patients who received the sirolimus-corticosteroids-oseltamivir combinatory treatment were also associated with improved clinical outcomes with respect to hypoxia, MODS, and duration of ventilator use (5). In our case, the acute symptoms and abnormalities in the thoracic radiographic findings progressed rapidly. The NAIs and ECMO did not result in symptomatic improvement, positive thoracic imaging findings, or viral titer relief until the prescription of sirolimus. Therefore, we propose that sirolimus might be a novel and practical therapeutic approach to severe H5N6-associated pneumonia in humans.
It is well-established that inflammation promotes the recruitment of immune cells during viral infections, while uncontrolled and exacerbated inflammation is associated with systemic edema and extensive tissue damage (11). One autopsy report found that when compared with pH1N1 fatal infection, H5N6 infection caused a more exacerbated immune response involving overt pulmonary inflammation (12). Similarly, Bi et al. tested 48 cytokines/chemokines and reported that H5N6 patients had significantly higher levels of cytokines/chemokines than those of healthy controls, H7N9 patients, and pH1N1 patients in 21, 11, and 5 parameters, respectively (13). In addition, the non-surviving H5N6 patients had higher concentrations of both pro-inflammatory and anti-inflammatory cytokines/chemokines than those of the survivors (13).
Sirolimus plays an essential role in both the innate and adaptive immune responses by inhibiting antibody production, B cell development, and T lymphocyte activation and proliferation that occur in response to antigenic and cytokine [interleukin (IL)-2,IL-4, and IL-15] stimulation (14). Keating et al. showed that sirolimus treatment during primary H3N2 virus infection protected mice against a lethal, heterosubtypic secondary infection with influenza virus subtype H5N1, H7N9, or H1N1 (15). Moreover, sirolimus reduced class switching in B cells and altered the specificity patterns of immunoglobulins G (IgG) and M (IgM), which yielded a unique repertoire of antibodies (15). In addition, sirolimus may ameliorate the immunopathological injury to the lung by modulating dysregulated pro-inflammatory and anti-inflammatory cytokine production (10,16,17). In a study of H1N1 viral infection by Jia et al., the groups receiving delayed oseltamivir-plus-sirolimus combinatory treatment exhibited significantly decreased concentrations of various pro-inflammatory cytokines and chemokines, as well as reduced inflammatory cell infiltration in lung tissue and BALF, in comparison with the untreated control or monotherapy groups (10). Sirolimus has also been suggested to optimize the treatment of coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (18,19).
Our case is the first to report the effectiveness and safety of sirolimus and NAIs in improving clinical outcomes of patient with severe H5N6 infection. However, there are some limitations. First, we did not assess the immunologic impact, such as levels of serum or bronchoalveolar lavage cytokines/chemokines, during the infection or the intervention. Second, the role of corticosteroid could not be excluded, as low-dose systemic corticosteroid was also used in this patient. Studies without concomitant corticosteroid therapy should be conducted. To further investigate the effects of sirolimus and oseltamivir on the normalization of respiratory status, the changes to 10 cytokines/chemokines and pro-inflammatory mediators, viral clearance, and several other clinical endpoints in influenza patients, a new study will be performed by the Chinese University of Hong Kong (20).
In conclusion, the role of sirolimus in patients with severe viral (including the highly pathogenic AIV and SARS-CoV-2) pneumonia is a promising direction for further investigation.
Acknowledgments
The authors would like to thank Professor Mengfeng Li of Southern Medical University and Professor Erwei Song of Sun Yat-sen Memorial Hospital, Sun Yat-sen University, for their contribution to the successful treatment of this patient.
Funding: This work was supported in part by the Emergency Program for Guangzhou Regenerative Medicine and Health Guangdong Laboratory of China (No. 2020GZR110106003) and the Tencent Charity Foundation of China.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-704/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-704/coif). The authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (No. SYSEC-KY-KS-2018-123). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sanders S, editors. FluTrackers.com. FluTrackers H5N6 Cumulative Case List. [cited 2021. 12. 8]. Available online: https://flutrackers.com/forum/forum/china-h5n1-h5n8-h5n6-h5n3-h5n2-h10n8-outbreak-tracking/723926-flutrackers-h5n6-cumulative-case-list
- Li H, Li Q, Li B, et al. Continuous Reassortment of Clade 2.3.4.4 H5N6 Highly Pathogenetic Avian Influenza Viruses Demonstrating High Risk to Public Health. Pathogens 2020;9:670. [Crossref] [PubMed]
- Louie JK, Yang S, Acosta M, et al. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin Infect Dis 2012;55:1198-204. [Crossref] [PubMed]
- Kuss-Duerkop SK, Wang J, Mena I, et al. Influenza virus differentially activates mTORC1 and mTORC2 signaling to maximize late stage replication. PLoS Pathog 2017;13:e1006635. [Crossref] [PubMed]
- Wang CH, Chung FT, Lin SM, et al. Adjuvant treatment with a mammalian target of rapamycin inhibitor, sirolimus, and steroids improves outcomes in patients with severe H1N1 pneumonia and acute respiratory failure. Crit Care Med 2014;42:313-21. [Crossref] [PubMed]
- Bonilla-Aldana DK, Aguirre-Florez M, Villamizar-Peña R, et al. After SARS-CoV-2, will H5N6 and other influenza viruses follow the pandemic path? Infez Med 2020;28:475-85. [PubMed]
- Shin YK, Liu Q, Tikoo SK, et al. Effect of the phosphatidylinositol 3-kinase/Akt pathway on influenza A virus propagation. J Gen Virol 2007;88:942-50. [Crossref] [PubMed]
- Smallwood HS, Duan S, Morfouace M, et al. Targeting Metabolic Reprogramming by Influenza Infection for Therapeutic Intervention. Cell Rep 2017;19:1640-53. [Crossref] [PubMed]
- Murray JL, McDonald NJ, Sheng J, et al. Inhibition of influenza A virus replication by antagonism of a PI3K-AKT-mTOR pathway member identified by gene-trap insertional mutagenesis. Antivir Chem Chemother 2012;22:205-15. [Crossref] [PubMed]
- Jia X, Liu B, Bao L, et al. Delayed oseltamivir plus sirolimus treatment attenuates H1N1 virus-induced severe lung injury correlated with repressed NLRP3 inflammasome activation and inflammatory cell infiltration. PLoS Pathog 2018;14:e1007428. [Crossref] [PubMed]
- Wang Z, Loh L, Kedzierski L, et al. Avian Influenza Viruses, Inflammation, and CD8(+) T Cell Immunity. Front Immunol 2016;7:60. [Crossref] [PubMed]
- Gao R, Pan M, Li X, et al. Post-mortem findings in a patient with avian influenza A (H5N6) virus infection. Clin Microbiol Infect 2016;22:574.e1-5. [Crossref] [PubMed]
- Bi Y, Tan S, Yang Y, et al. Clinical and Immunological Characteristics of Human Infections With H5N6 Avian Influenza Virus. Clin Infect Dis 2019;68:1100-9. [Crossref] [PubMed]
- Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 2009;9:324-37. [Crossref] [PubMed]
- Keating R, Hertz T, Wehenkel M, et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat Immunol 2013;14:1266-76. [Crossref] [PubMed]
- Wang H, Brown J, Gu Z, et al. Convergence of the mammalian target of rapamycin complex 1- and glycogen synthase kinase 3-β-signaling pathways regulates the innate inflammatory response. J Immunol 2011;186:5217-26. [Crossref] [PubMed]
- Alsuwaidi AR, George JA, Almarzooqi S, et al. Sirolimus alters lung pathology and viral load following influenza A virus infection. Respir Res 2017;18:136. [Crossref] [PubMed]
- Husain A, Byrareddy SN. Rapamycin as a potential repurpose drug candidate for the treatment of COVID-19. Chem Biol Interact 2020;331:109282. [Crossref] [PubMed]
- Zhou Y, Hou Y, Shen J, et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov 2020;6:14. [Crossref] [PubMed]
- Adjunctive sirolimus and oseltamivir versus oseltamivir alone for treatment of influenza. [cited 2021. 12. 8]. Available online: https://clinicaltrials.gov/ct2/show/NCT03901001?term=sirolimus&cond=Influenza%2C+Human&draw=2&rank=1
(English Language Editor: C. Gourlay)


