
Ultrasound score combined with liver stiffness measurement by sound touch elastography for staging liver fibrosis in patients with chronic hepatitis B: a clinical prospective study
Introduction
Liver fibrosis staging is crucial for managing clinical treatment of the disease and predicting treatment response (1). A variety of methods have been developed to stage liver fibrosis in patients with chronic hepatitis B (CHB) (2); however, most of these methods are limited by complications or inaccuracies (3-5). The gold standard for liver fibrosis staging, liver biopsy, is an invasive method that can result in complications such as hemorrhage, infection, and pain (6,7), leading to patient reluctance in consenting to the procedure. Therefore, noninvasive methods such as liver ultrasound (US) and ultrasound elastography (USE) have been proposed as an alternative means of assessing liver fibrosis.
In clinical practice, abdominal US is used in the initial workup for patients with chronic viral hepatitis. Studies have shown that US image features such as an uneven or undulating liver surface; a heterogeneous echotexture of the liver parenchyma; and changes in blood vessel diameters, blood flow velocity, and spleen size are correlated with severe liver fibrosis or cirrhosis (8,9). Accordingly, several US scoring systems and guidelines have been developed for radiologists to noninvasively stage liver fibrosis in US images. A previous study has shown that the diagnostic performance of US scores by radiologists typically have low sensitivity and high specificity (10).
USE is now widely recognized as a reliable method for the assessment of liver fibrosis (11-13). Current USE methods include transient elastography (TE) and sound touch elastography (STE). As the first developed USE method, TE has been widely used in clinical practice (14). However, liver stiffness measurements (LSMs) produced by TE for fibrosis assessment may be influenced by patient-dependent factors such as obesity, ascites, resulting in an unreliable diagnosis of fibrosis stage (15,16). With the continuous development of USE techniques, the limitations of TE have been mitigated by the more advanced STE approach, which assesses tissue stiffness by directly measuring the velocity of shear waves generated by the US and calculating Young’s modulus to produce a stiffness measurement. A recent study has shown that STE is superior to TE in assessing liver fibrosis in CHB, while the diagnosis with the combined use of these two techniques is superior to STE or TE alone (17).
This study aimed to assess the diagnostic performance of US score and LSM of STE in diagnosing liver fibrosis stages and to investigate whether combining these methods would improve liver fibrosis staging. We present the following article in accordance with the STARD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-505/rc).
Methods
Subjects
From September 2018 to January 2020, consecutive patients with CHB virus infections were prospectively enrolled in our study. The inclusion criteria were as follows: (I) patients were aged between 18 and 65 years old; (II) the hepatitis B surface antigen was positive for longer than 6 months; (III) in the past 6 months, alanine transaminase and aspartate aminotransferase levels were less than 2 times the normal upper limit; and (IV) patient’s body mass index (BMI) between 18.5 and 31.0. The exclusion criteria were as follows: (I) the time interval between liver biopsy and USE was more than 1 week; (II) patients had other chronic liver diseases, such as alcoholic liver disease or autoimmune hepatitis; (III) patients had jaundice; and (IV) LSM operation failure. Healthy volunteers were also recruited to serve as a control group. The inclusion criteria for the control group were as follows: (I) patients were aged between 18 and 65 years old; (II) patients had a BMI between 18.5 and 31.0; (III) patients had no historical record of liver disease; (IV) the blood biochemical indexes related to liver function were normal; and (V) patients had no abnormal signs during conventional US examination. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee Board of Shenzhen Third People’s Hospital, Shenzhen, Guangdong Province, China [No. (2018) 02-202-01]. Written informed consent was received from all enrolled patients.
US examination
Within 1 week of the liver biopsy, each subject underwent a US examination after fasting overnight. A single skilled sonographer (C.D., with 17 years of US experience), who was blinded to the subject’s clinical details, performed the examinations on a US system (Resona 7, Mindray, Shenzhen, China) with the following 2 probes: a linear-array probe (L11-3U, 6.2–10.8 MHz) and a convex-array probe (SC6U-1, 1–6 MHz). The linear-array probe was used to capture US images of the liver surface (for liver surface smoothness evaluation by radiologists), while the convex-array probe was used to collect images of the liver parenchyma and spleen (for texture comparison and analysis by radiologists). The subject was instructed to maintain a supine position during US examination with both hands extended to the head. The images of the liver surface, liver parenchyma, and spleen were scanned in static B-mode, following previous studies (18,19).
LSM procedure
The same sonographer conducted an LSM using the same US system with the convex-array probe (SC6U-1, 1–6 MHz). The procedure for LSM was as follows. After the US examination, the subject was requested to maintain a supine position with the right arm at maximum abduction. Then, the sonographer changed the scanning mode of the US system from conventional B-mode to STE. A circled region of interest (ROI) with a diameter of 20 mm was set within a 4 cm × 3 cm sampling window in the right liver, taking care to avoid large vessels and bile ducts, and the stiffness value of the tissue in the circled ROI was recorded. The stiffness value was obtained by calculating Young’s modulus (20) of the tissue using the following equation: E=3G=3ρCs2 (in kilopascal, kPa), where G is the shear modulus that quantifies the deformation of the medium, ρ is the density of the tissue, and Cs is the speed of the shear wave. As shear waves propagate faster in harder tissues, the corresponding Young’s modulus of the harder tissues is larger. To control the quality of LSM by STE, a motion stability (M-STB) index and a reliability (RLB) map (21) were used as recording indicators. The M-STB index is denoted by stars (with the highest M-STB shown by five green stars), while the RLB map changes from purple to green (with the latter indicating the highest RLB). The liver stiffness value was recorded when the following conditions were met: the M-STB index was above 4 stars and the RLB index derived from the RLB map was above 90%. The LSM process was repeated 5 times at the same location of the right liver to get 5 LSM values, the median of which was the final stiffness value.
Pathological examination
Within 1 week of the US examination and LSM, each patient with CHB underwent a US-guided percutaneous liver biopsy of the right lobe using a 16-gauge biopsy needle [MC1616 (16 G, 16 cm), BARD, Tempe, AZ, USA]. The biopsied liver tissue strip, which was 10–20 mm in length and contained at least 6 portal areas, was made into paraffin sections and stained with Sirius Red (S1020, Wuhan Haotian Bioscience Technology Co., Ltd., Hubei, China). Two pathologists, who were blinded to the US and LSM results, independently evaluated the specimens according to the METAVIR scoring system (22) [normal (F0), no hepatic fibrosis; mild fibrosis (F1), fibrosis in the manifold but no fibrous septa; advantaged fibrosis (F2), fibrosis in the portal manifold with a small amount of fibrous septa; severe fibrosis (F3), a large number of fibrous septa but no cirrhosis; and cirrhosis (F4)]. Any discrepancies between the pathologists’ interpretations were resolved by discussion. The pathological results of liver biopsy (6,7) served as the reference standard for assessing the performance of noninvasive diagnosing markers.
US score
A US scoring system was developed to evaluate the degree of liver fibrosis. The system included the following evaluations: the smoothness of the liver capsule surface (0= a smooth surface, 1= an uneven or wavy surface); the homogeneity of the liver parenchyma, assessed by comparing the texture of the liver with that of the spleen (0= homogeneous parenchyma, 1= slightly heterogeneous parenchyma, 2= significant heterogeneous parenchyma, 3= coarse liver parenchyma with an irregular pattern). Two radiologists (C.D. and C.F) with at least 15 years of US experience, who were blinded to the results of the STE and the pathological evaluations, independently reviewed and scored the B-mode images. Consensus was obtained through discussion in the case of discrepant interpretations. The final US score was calculated as the sum of the liver capsule surface scores and the parenchymal texture scores, with values ranging from 0 to 4.
Combination of US score and LSM
In addition to assessing liver fibrosis with only US score or LSM, we also assessed liver fibrosis by combining these 2 diagnostic markers. The coefficients for an optimal linear combination (LC) of the 2 markers were computed using the Fisher discriminant analysis (FDA) method (23,24), which maximizes interclass distance while minimizing intraclass dispersion. When the diagnostic markers to be combined are normal-distributed with proportional covariance matrices, the LC yielded by FDA can provide an optimal diagnostic performance with maximum sensitivities over the entire range of specificity (25).
Statistical analysis
Our study used 3 diagnostic markers for liver fibrosis assessment: US score, LSM, and the LC of the two. Spearman correlation was used to analyze the correlation between each marker and the histological findings. The diagnostic performance of each marker was assessed by the receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC). The Youden index (26), defined as J = sensitivity + specificity − 1, was maximized to identify the optimal cutoff value on the ROC curve, as well as the accuracy, sensitivity, and specificity corresponding to the cutoff value. 95% confidence intervals (CIs) for accuracy, sensitivity, and specificity were computed using the Clopper-Pearson method (27). Similarly to a previous study (28), we used the binomial test to measure the difference in accuracy, sensitivity, and specificity and employed the DeLong test (29) to compare the resultant ROC curves. All statistical tests were performed using MATLAB version R2019a (The MathWorks Inc., Natick, MA, USA). The P values were 2 sided, and values less than 0.05 were considered to be statistically significant.
Results
Subject characteristics
A total of 330 subjects were enrolled in this prospective study between September 2018 and January 2020. According to the inclusion and exclusion criteria, 242 patients with CHB and 49 healthy volunteers were finally included (Figure 1). The 49 healthy volunteers served as a control group (F0), while the 242 patients with CHB were stratified into 4 groups with different fibrosis stages ranging from F1 to F4 according to the pathological results of the liver biopsy. Detailed characteristics of the included subjects are presented in Table 1. The raw clinical information and reference standard results are available upon reasonable request to the corresponding authors.
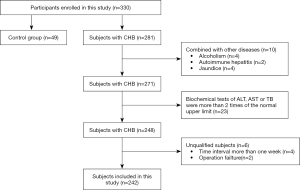
Table 1
| Variables | Value |
|---|---|
| No. of subjects | 291 |
| Age (years)* | 39.07±9.45 |
| Gender (male/female) | 192/99 |
| Body max index (kg/m2)* | 22.40±4.22 |
| platelet count (103/mm3)# | 193.50 (146.50–230.00) |
| AST level (IU/L)# | 23.00 (19.00–29.00) |
| ALT level (IU/L)# | 23.00 (16.00–34.00) |
| Total bilirubin level (IU/L)# | 13.60 (10.05–18.10) |
| US score (0/1/2/3/4) | 49/113/39/23/67 |
| Subjects in control group | 49 |
| METAVIR score (F1/F2/F3/F4) | 84/61/49/48 |
Integer numbers in the right column are the number of patients. *, data are represented by mean and standard deviations; #, data are medians with the interquartile range in parentheses. AST, aspartate aminotransferase; ALT, alanine aminotransferase; US, ultrasonography.
LC model
An LC model was derived by using the FDA method to fuse US score and LSM for noninvasive staging of liver fibrosis. This model was as follows:
The coefficient assigned to LSM was smaller than that assigned to US score, as the liver stiffness values represented in kPa were larger than the US score.
Correlation analysis
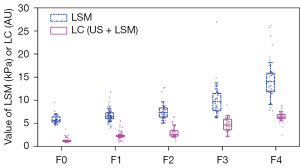
The US score, LSM, and LC marker produced by the LC model were positively correlated to the fibrosis stages, with Spearman correlation coefficients of r=0.825 (P<0.001), r=0.771 (P<0.001) and r=0.846 (P<0.001), respectively. The correlation results showed that the LC marker, which combined US score and LSM, correlated to the fibrosis stages better than did US score or LSM alone. Figure 2 plots the median values of LSM and the LC marker under different fibrosis stages determined via histological evaluation.
Diagnostic performance comparisons
The cutoff values and the diagnostic performance of US score, LSM, and the LC marker are summarized in Table 2. The cutoff values of the LC marker gradually increased from 1.97 AU (arbitrary unit) to 5.06 AU for diagnosing the fibrosis stages of ≥F1, ≥F2, ≥F3, and =F4. Similar patterns of the cutoff values in diagnosing the fibrosis stages of ≥F1, ≥F2, ≥F3, and =F4 could be observed for LSM (increased from 6.06 to 11.24 kPa) and US score (increased from 1 to 3).
Table 2
| Degrees | US score | LSM | LC (US + LSM) |
|---|---|---|---|
| ≥F1 (cutoff) | 1 | 6.06 kPa | 1.97 AU |
| Accuracy | 0.924 [269/291] (0.888–0.952, P=0.601) | 0.832 [242/291] (0.784–0.873, P<0.001) | 0.914 [266/291] (0.876–0.944) |
| Sensitivity | 0.959 [232/242] (0.925–0.980, P=0.031) | 0.855 [207/242] (0.805–0.897, P<0.001) | 0.921 [223/242] (0.880–0.952) |
| Specificity | 0.755 [37/49] (0.611–0.867, P=0.015) | 0.714 [35/49] (0.567–0.834, P=0.002) | 0.878 [43/49] (0.752–0.954) |
| AUC | 0.916 (0.883–0.948, P=0.014) | 0.858 (0.812–0.948, P<0.001) | 0.943 (0.917–0.948) |
| ≥F2 (cutoff) | 2 | 7.11 kPa | 2.45 AU |
| Accuracy | 0.811 [236/291] (0.761–0.854, P=0.347) | 0.804 [234/291] (0.754–0.848, P=0.210) | 0.832 [242/291] (0.784–0.873) |
| Sensitivity | 0.734 [116/158] (0.658–0.801, P=0.019) | 0.797 [126/158] (0.726–0.857, P=0.685) | 0.810 [128/158] (0.740–0.868) |
| Specificity | 0.902 [120/133] (0.839–0.947, P=0.171) | 0.812 [108/133] (0.735–0.875, P=0.137) | 0.857 [114/133] (0.786–0.912) |
| AUC | 0.875 (0.835–0.915, P<0.001) | 0.867 (0.826–0.915, P=0.006) | 0.906 (0.871–0.915) |
| ≥F3 (cutoff) | 2 | 8.04 kPa | 3.34 AU |
| Accuracy | 0.856 [249/291] (0.810–0.894, P=0.045) | 0.856 [249/291] (0.810–0.894, P=0.045) | 0.893 [260/291] (0.852–0.926) |
| Sensitivity | 0.948 [92/97] (0.884–0.983, P=0.557) | 0.845 [82/97] (0.758–0.911, P=0.005) | 0.928 [90/97] (0.857–0.970) |
| Specificity | 0.809 [157/194] (0.747–0.862, P=0.008) | 0.861 [167/194] (0.804–0.906, P=0.512) | 0.876 [170/194] (0.822–0.919) |
| AUC | 0.934 (0.898–0.969, P=0.001) | 0.930 (0.894–0.969, P<0.023) | 0.953 (0.923–0.969) |
| =F4 (cutoff) | 3 | 11.24 kPa | 5.06 AU |
| Accuracy | 0.842 [245/291] (0.795–0.882, P=0.001) | 0.914 [266/291] (0.876–0.944, P=0.619) | 0.904 [263/291] (0.864–0.935) |
| Sensitivity | 0.958 [46/48] (0.857–0.995, P=1.000) | 0.896 [43/48] (0.773–0.965, P=0.049) | 0.958 [46/48] (0.857–0.995) |
| Specificity | 0.819 [199/243] (0.765–0.865, P=0.001) | 0.918 [223/243] (0.876–0.949, P=0.253) | 0.893 [217/243] (0.847–0.929) |
| AUC | 0.918 (0.864–0.973, P<0.001) | 0.958 (0.918–0.973, P=0.778) | 0.961 (0.922–0.973) |
P values (binomial test for accuracy, sensitivity, and specificity, and DeLong test for AUC) were obtained by comparing the results of LC to those of US score and LSM. Data in square brackets are raw data, and data in parenthesis are 95% CI and P values when applicable. US, ultrasonography; LSM, liver stiffness measurement; LC, linear combination of US score and LSM; AUC, area under the receiver operating characteristic curve; AU, arbitrary unit.
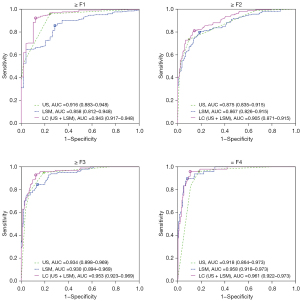
The AUC values offered by using the LC marker (US score + LSM; Table 2) to diagnose ≥F1, ≥F2, ≥F3, and =F4 were, respectively, 0.943 (95% CI: 0.917–0.948), 0.906 (0.871–0.915), 0.953 (0.923–0.969), and 0.961 (0.922–0.973). These values were better than the corresponding AUC values of US score, which were 0.916 (0.883–0.948, P=0.014), 0.875 (0.835–0.915, P<0.001), 0.934 (0.898–0.969, P=0.001), and 0.918 (0.864–0.973, P<0.001), or those of LSM, which were 0.858 (0.812–0.948, P<0.001), 0.867 (0.826–0.915, P=0.006), 0.930 (0.894–0.969, P<0.023), and 0.958 (0.918–0.973, P=0.778). In contrast to US score, the LC marker’s AUC values improved by 2.7%, 3.1%, 1.9%, and 4.3% for, ≥F1, ≥F2, ≥F3, and =F4, respectively, and these improvements were statistically significant (all P values were <0.05).
The increased AUC percentages of the LC marker when compared with LSM were 9.5, 3.9, 2.3, and 0.3 for ≥F1, ≥F2, ≥F3, and =F4, respectively, with all P values <0.05 except for =F4. A comparison of the AUC values of US score and LSM showed that US score was superior in diagnosing mild liver fibrosis (≥F1), while LSM had the advantage of predicting liver cirrhosis (=F4). The LC marker, generated by the LC of US score and LSM, inherited the advantages of these 2 diagnostic markers, and thus its overall performance in predicting liver fibrosis stage was superior to that of the diagnostic markers alone.
The ROC curves corresponding to the diagnostic markers are shown in Figure 3. In the case of ≥F1, the ROC curve of US score was better than that of LSM, whereas for =F4, the curve of LSM was better than that of US score. With respect to ≥F2 and ≥F3, US score and LSM provided AUC curves with similar AUC values. By combining US score and LSM, the LC marker produced ROC curves with the largest AUC values for all cases in predicting ≥F1, ≥F2, ≥F3, and =F4.
Discussion
When evaluated using low frequency and high frequency probes, the edge, surface, and parenchymal texture of livers with mild fibrosis correlate with pathology findings (Spearman’s r=0.667) with a diagnostic accuracy of 79% (30). As liver fibrosis develops, liver parenchymal echo patterns change from normal uniformity to severe coarseness. This change has been verified by pathological results (31). In the early stage of liver fibrosis, although a small amount of hepatic cell degeneration and point necrosis forms, the liver parenchyma echo is still uniform (score 0, for parenchymal texture) or slightly heterogeneous (score 1, for parenchymal texture), and the liver capsule is smooth (score 0, for capsule surface). When the liver is obviously damaged, the parenchyma echo shows significant heterogeneity (score 2, for parenchymal texture), but the structure of the liver lobule remains intact, and the liver capsule is still smooth (score 0, for capsule surface). As fibrosis develops, large areas of hepatocyte necrosis and fibrous septa are formed, resulting in a liver parenchymal echo that is obviously nonuniform and coarse with an irregular pattern (score 3, for parenchymal texture). The evaluation of liver fibrosis by US scoring is based on the pathological changes that occur during liver fibrosis progression. Thus, it is reasonable to combine US scores with other markers when seeking to improve the diagnosis of liver fibrosis.
In this study, a US scoring system was established based on the evaluation of US features with respect to the following two characteristics: the texture of the liver parenchyma and the smoothness of the liver capsule. Other US image features, including the diameter of the portal and splenic veins, spleen texture, and blood biochemical indicators, show statistical significance mainly in severe liver fibrosis and cirrhosis, which indicates that the associated changes usually occur in the cirrhosis period and reflect the presence of severe fibrosis or cirrhosis (32-34). Therefore, these features were excluded from our US scoring system so as not to degrade the performance of the combined diagnostic marker in predicting different stages of liver fibrosis.
The results of our study showed that STE LSM combined with US score offered excellent diagnostic performance in staging liver fibrosis. The contribution of fusing LSM and US score to improving the diagnostic performance of liver fibrosis can be explained as follows. First, two-dimensional (2D) US images show typical signs of fibrosis, such as obvious change of liver parenchymal echo and irregular pattern (35). Second, the characteristics of STE are similar to those of 2D shear wave imaging (36), and the latter had been proven to have good interobserver and interdevice variability (37). Third, previous studies have shown that the extent of hepatic fibrosis detected by biopsy correlates well with LSM and US results (20,38). As hepatic fibrosis progresses, the severity of structural changes observed in US images correlates positively with LSM values; therefore, the combination of these 2 diagnosis markers can provide more details, resulting in a better diagnosis performance. Finally, the marker values provided by the LC of US score and LSM, LC = 0.9829 × US score + 0.1823 × LSM, showed a smaller range overlap between adjacent fibrosis stages than those of LSM alone (as shown in Figure 2), which contributed to the better performance of the cutoff values in diagnosing various fibrosis stages. Therefore, the prediction accuracy improvements made by the LC marker may enhance the confidence of radiologists in diagnosing liver fibrosis.
Our study has some limitations. First, US score may be affected by the experience and subjectivity of the technician. Second, our study focused on patients with CHB whose transaminases were less than 2.0 times the upper limit of normal. The combined diagnostic marker was established based on this standard. In the future, we may expand the sample size to verify the diagnostic performance and limitations of this combined marker in other conditions, such as in the case of patients with abnormal transaminases.
Conclusions
The LC diagnostic marker developed by the LC of US score and STE LSM showed good correlation with pathological findings. The overall performance of the LC marker was better than that of US score or STE LSM alone. Therefore, an LC marker using an LC of US score and STE LSM may be a promising indicator for noninvasive liver fibrosis staging.
Acknowledgments
We would like to thank Xiang-Mei Zhang and Xiao-Hua Le (Ph.D., National Clinical Research Center for Infectious Disease, State Key Discipline of Infectious Disease, Shenzhen Third People’s Hospital, Second Hospital Affiliated to Southern University of Science and Technology, China) for their assistance in the work of liver histology assessment.
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 82172563, 81871429, and 61901282); Key-Area Research and Development Program of Guangdong Province (No. 2020B1111130002); the clinical research project of Shenzhen Third People’s Hospital (No. G2022018).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-505/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-505/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-505/coif). All authors report that this work was supported by the National Natural Science Foundation of China (Nos. 82172563, 81871429, and 61901282); Key-Area Research and Development Program of Guangdong Province (No. 2020B1111130002); and the clinical research project of Shenzhen Third People’s Hospital (No. G2022018). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee Board of Shenzhen Third People’s Hospital, Shenzhen, Guangdong Province, China [No. (2018) 02-202-01]. Written informed consent was received from all enrolled patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seto WK, Lo YR, Pawlotsky JM, et al. Chronic hepatitis B virus infection. Lancet 2018;392:2313-24. [Crossref] [PubMed]
- Gatos I, Tsantis S, Spiliopoulos S, et al. A Machine-Learning Algorithm Toward Color Analysis for Chronic Liver Disease Classification, Employing Ultrasound Shear Wave Elastography. Ultrasound Med Biol 2017;43:1797-810. [Crossref] [PubMed]
- Park HS, Choe WH, Han HS, et al. Assessing significant fibrosis using imaging-based elastography in chronic hepatitis B patients: Pilot study. World J Gastroenterol 2019;25:3256-67. [Crossref] [PubMed]
- Herrmann E, de Lédinghen V, Cassinotto C, et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology 2018;67:260-72. [Crossref] [PubMed]
- Petroff D, Blank V, Newsome PN, et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol 2021;6:185-98. [Crossref] [PubMed]
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344:495-500. [Crossref] [PubMed]
- Dong B, Huang S, Chang J, et al. Comparison of Sound Touch Elastography, Sound Touch Quantify, and 4 Serum Fibrosis Indexes for the Diagnosis of Liver Fibrosis in Patients With Chronic Hepatitis B. Ultrasound Q 2021;37:123-8. [Crossref] [PubMed]
- Salvatore V, Borghi A, Peri E, et al. Relationship between hepatic haemodynamics assessed by Doppler ultrasound and liver stiffness. Dig Liver Dis 2012;44:154-9. [Crossref] [PubMed]
- Talwalkar JA, Kurtz DM, Schoenleber SJ, et al. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2007;5:1214-20. [Crossref] [PubMed]
- Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008;134:960-74. [Crossref] [PubMed]
- Tsochatzis EA, Gurusamy KS, Ntaoula S, et al. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol 2011;54:650-9. [Crossref] [PubMed]
- Dong DR, Hao MN, Li C, et al. Acoustic radiation force impulse elastography, FibroScan®, Forns' index and their combination in the assessment of liver fibrosis in patients with chronic hepatitis B, and the impact of inflammatory activity and steatosis on these diagnostic methods. Mol Med Rep 2015;11:4174-82. [Crossref] [PubMed]
- Chon YE, Choi EH, Song KJ, et al. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One 2012;7:e44930. [Crossref] [PubMed]
- Xiao H, Shi M, Xie Y, et al. Comparison of diagnostic accuracy of magnetic resonance elastography and Fibroscan for detecting liver fibrosis in chronic hepatitis B patients: A systematic review and meta-analysis. PLoS One 2017;12:e0186660. [Crossref] [PubMed]
- Wang K, Lu X, Zhou H, et al. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut 2019;68:729-41. [Crossref] [PubMed]
- Hung CH, Lu SN, Wang JH, et al. Correlation between ultrasonographic and pathologic diagnoses of hepatitis B and C virus-related cirrhosis. J Gastroenterol 2003;38:153-7. [Crossref] [PubMed]
- Wang JH, Changchien CS, Hung CH, et al. FibroScan and ultrasonography in the prediction of hepatic fibrosis in patients with chronic viral hepatitis. J Gastroenterol 2009;44:439-46. [Crossref] [PubMed]
- Ophir J, Céspedes I, Ponnekanti H, et al. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging 1991;13:111-34. [Crossref] [PubMed]
- Barr RG, Wilson SR, Rubens D, et al. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology 2020;296:263-74. [Crossref] [PubMed]
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289-93. [Crossref] [PubMed]
- Fisher RA. The use of multiple measurements in taxonomic problems. Annals of Eugenics 1936;7:179-88. [Crossref]
- Fukunaga K. Introduction to statistical pattern recognition. Elsevier; 2013.
- Su JQ, Liu JS. Linear combinations of multiple diagnostic markers. J Am Stat Assoc 1993;88:1350-5. [Crossref]
- Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32-5. [Crossref] [PubMed]
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404-13. [Crossref]
- Li X, Zhang S, Zhang Q, et al. Diagnosis of thyroid cancer using deep convolutional neural network models applied to sonographic images: a retrospective, multicohort, diagnostic study. Lancet Oncol 2019;20:193-201. [Crossref] [PubMed]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. [Crossref] [PubMed]
- Lin Z, Liang J, Zhu J, et al. Diverse correlations between fibrosis-related factors and liver stiffness measurement by transient elastography in chronic hepatitis B. Eur J Gastroenterol Hepatol 2018;30:217-25. [Crossref] [PubMed]
- Cheng L, Chen Y, Xiao R, et al. Evaluation of hepatic fibrosis by ultrasonic acoustic structure quantification. Medicine (Baltimore) 2019;98:e16533. [Crossref] [PubMed]
- Zhou H, Long J, Hu H, et al. Liver stiffness and serum markers for excluding high-risk varices in patients who do not meet Baveno VI criteria. World J Gastroenterol 2019;25:5323-33. [Crossref] [PubMed]
- Ozturker C, Karagoz E, Mutlu H. How useful is ARFI elastography for predicting the significant fibrosis and compensated liver cirrhosis? Med Ultrason 2016;18:131. [Crossref] [PubMed]
- Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013;33:1138-47. [Crossref] [PubMed]
- Memon SK. Ultrasonographic Findings of Liver in Chronic Liver Disease and its Complications and Their Association with the Duration of the Disease. J Coll Physicians Surg Pak 2017;27:127-30. [PubMed]
- Bota S, Paternostro R, Etschmaier A, et al. Performance of 2-D shear wave elastography in liver fibrosis assessment compared with serologic tests and transient elastography in clinical routine. Ultrasound Med Biol 2015;41:2340-9. [Crossref] [PubMed]
- Gatos I, Drazinos P, Yarmenitis S, et al. Comparison of Sound Touch Elastography, Shear Wave Elastography and Vibration-Controlled Transient Elastography in Chronic Liver Disease Assessment using Liver Biopsy as the "Reference Standard". Ultrasound Med Biol 2020;46:959-71. [Crossref] [PubMed]
- Udompap P, Sukonrut K, Suvannarerg V, et al. Prospective comparison of transient elastography, point shear wave elastography, APRI and FIB-4 for staging liver fibrosis in chronic viral hepatitis. J Viral Hepat 2020;27:437-48. [Crossref] [PubMed]
- Ye J, Wang W, Feng S, et al. Precise fibrosis staging with shear wave elastography in chronic hepatitis B depends on liver inflammation and steatosis. Hepatol Int 2020;14:190-201. [Crossref] [PubMed]
- Liu J, Ren W, Ai H, et al. Acoustic Structure Quantification Versus Point Shear Wave Speed Measurement for the Assessment of Liver Fibrosis in Viral Hepatitis B. Ultrasound Med Biol 2018;44:1177-86. [Crossref] [PubMed]
(English Language Editor: C. Gourlay)


